
In publishing its proposed rule on disclosing drug prices just three weeks before the mid-term Congressional elections, it’s not a big stretch to see the move by the Trump administration as designed for short-term political purposes.

In publishing its proposed rule on disclosing drug prices just three weeks before the mid-term Congressional elections, it’s not a big stretch to see the move by the Trump administration as designed for short-term political purposes.
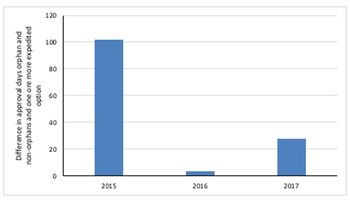
Leela Barham looks at whether there is a boost to the speed of FDA approval when a drug not only secures one or more of the FDA’s expedited development and review methods, but also when they are also designated an orphan drug.
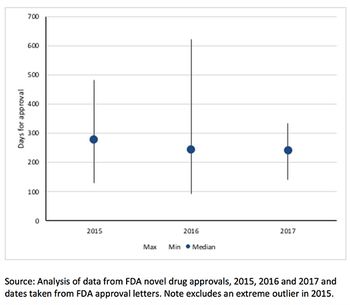
There’s been a lot of talk about speeding up drug approval at FDA. So just how fast can approval be? Leela Barham takes a look at the speed of approval for each of FDA's expedited development and review methods.
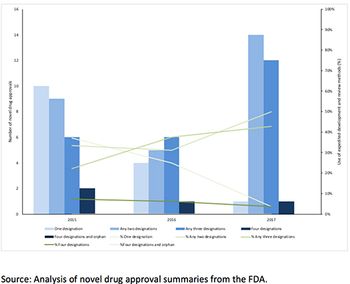
Leela Barham takes a look at the take-up of the existing options to speed up FDA approval.
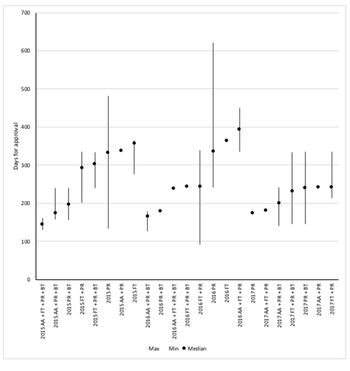
FDA offers four options to speed up approval and they can be used in combination. But just which of the many combinations offers the fastest approval? Leela Barham reports.


The call to reduce market entry times for life-saving new drug therapies is becoming more urgent. What constitutes best practice in managing translations in today’s regulatory affairs environment? Nancy Pollini reports.

CDER director plans to start implementation of new review practices by the end of 2018.

FDA is joining with other federal health agencies and the biomedical research community to advance the science, regulatory policies and reimbursement strategies to support innovative new medicines to combat infectious diseases.

A list of five essential features a building must have in order to be considered lab-ready.

With the rise in industry programs for developing biosimilars, tensions are high as innovator firms back strategies that appear designed to delay competition. Jill Wechsler reports.

New Action Plan aims to streamline development, but rebates and reimbursement block market access.

A wave of potentially impure drugs and APIs from China, India, and other foreign firms has prompted recalls and FDA warning letters.

The Senate has approved several multi-agency budget bills for the coming fiscal year that boost funding for FDA and the NIH – and include a contentious provision that requires biopharma companies to disclose product prices in direct-to consumer advertising.

A recently announced collaboration between EUnetHTA, WHO, and ISPOR brings home the fact that there is still no real agreement on a definition of HTA, writes Reflector.

Just as biopharma companies are mastering the complexities of the five-year-old federal Open Payments program, state governments are enacting a host of additional marketing and disclosure rules and restrictions on industry interactions with health care professionals.

While digital transformation in life sciences starts with digitization for compliance, it should be seen as a key lever of competitive advantage and success, writes Jaleel Shujath.

New Action Plan aims to streamline development, but rebates and reimbursement block market access.

DIA’s global chief executive talks with Pharm Exec about the current trajectory of patient-centric product development and the need to establish common pathways and metrics to best harness its benefits in everyday practice.

Artificial intelligence adoption by pharma companies in the UK demands a new, agile governance layer, write Tim Wright and Antony Bott.

Scott Gottlieb is proposing to flatten out FDA’s structure by having Center directors report directly to him, giving more authority to the agency’s six product regulatory Centers and to the Office of Regulatory Affairs.

Rising pressure to access less expensive prescription medicines available overseas is prompting the Trump administration to explore flexible import policies, reports Jill Wechsler.

Raman Sehgal talks to three industry leaders for their insights on the challenges faced across the life science sector and how the EMA needs to respond.

Streamlined clinical research, more guidance speed new cures to patients.

At this month's AMPLEXOR Life Sciences’ “Be The Expert” conference, Pharm Exec caught up with Steve Gens, who talked about the evolution of Regulatory Information Management (RIM) and offered some predictions for 2022.