
Pharmaceutical Executive
Pharma companies in Europe believe that it already takes too long for new medicines to reach patients. Separate bodies for efficacy and safety will lead to further delays.

Pharmaceutical Executive
Pharma companies in Europe believe that it already takes too long for new medicines to reach patients. Separate bodies for efficacy and safety will lead to further delays.
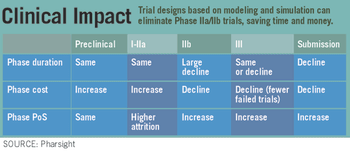
Pharmaceutical Executive
The use of a CUI leads development efforts to explre the most valuable region of treatment - not necessarily the the most efficacious.

Pharmaceutical Executive
In the drug safety debate, we're hearing plenty of potential solutions. They're smart and advance desirable goals. There's just one thing wrong with them: They don't solve the problem.

Pharmaceutical Executive
In an ideal world, an anti-counterfeit solution would provide protection throughout the supply chain, allow for easy product identification by physicians, pharmacists, and patients, be easily implemented without ongoing costs-and improve brand image and marketability while it's at it. Yet most current anti-counterfeiting measures involve packaging technologies such as holograms, inks, bar codes and radio frequency ID (RFID) that, although useful, cannot ensure the integrity of the pharmaceutical supply chain, because drugs do not remain in their original packaging. Legitimate repackaging regularly occurs in the pharmacy and elsewhere, and authentic packaging-recycled or stolen-can contain adulterated, counterfeited drugs.

Pharmaceutical Executive
Should policy makers expect 90 percent of seniors to enroll in PDPs, or will 75 percent be enough? Will the program have to keep costs down to $400 billion a year, or will spending be linked to savings elsewhere?

Pharmaceutical Executive
Pharm Exec talks to the newly appointed leaders of the industry's most influential advocacy organizations.