Are the Right Drugs Getting Faster FDA Approval?
Leela Barham continues her series of features exploring expedited FDA approvals with a look at the therapy areas and the overlap with orphan drug designations.
The heritage for introducing faster FDA approvals is to speed up access to treatments that address some of the most serious conditions. With more than half of novel drug approvals by the FDA in the last three years approved using one or more expedited approaches, are the right drugs getting faster approval? Leela Barham takes a look at the therapy areas and the overlap with orphan drug designations in another in a series of features exploring the expedited FDA approvals.
A focus on cancer
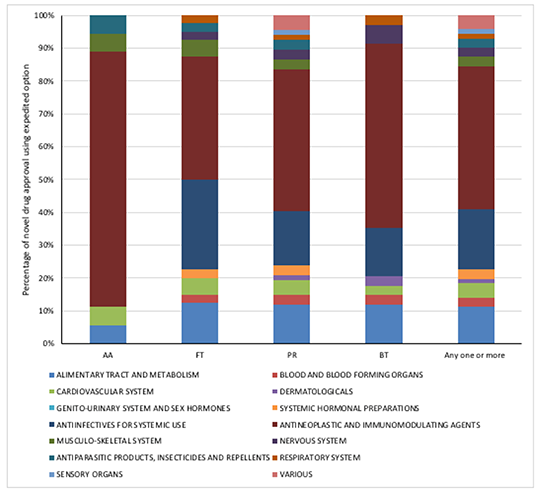
Drugs approved in the last three years using one or more FDA expedited option have been across a number of therapy areas, although cancer is particularly a focus for Accelerated Approval and accounts for almost half (43%) of all novel drug approvals that have used one or more expedited options. That means that there is a focus on a key disease area that is important to society: cancer is the second leading cause of death globally and it’s topped the list of American health concerns in surveys.
Figure 1: FDA novel drug approvals between 2015 and 2017, using an expedited development and review method by ATC class
Source: Analysis of FDA novel drug approvals data 2015, 2016 and 2017 and classified by ATC category.
Fast lanes for orphans
Special incentives have been put in place through the US Orphan Drug Act 1983 to encourage companies to develop orphan drugs: drugs to treat rare conditions. In the US companies have to show that the drug will treat a condition with fewer than 200,000 people in the US or drugs won’t be profitable within seven years of approval by the FDA. One of the benefits of the US Orphan Drug Act 1983 has been described by sociologist Carlos Novas as shifting the conduct of firms and others in directions considered socially – and economically – desirable.
It’s also a rare disease that Trump has referenced in his criticism of FDA for both being slow but also that the FDA processes keeps too many advances from reaching those in need. Trump highlighted the case of Megan Crowley who has Pompe disease. In her case, her father set up a biotech to develop a treatment. FDA approval came in 9 months but Crowley’s father had given her and her brother the enzyme replacement therapy three years before FDA approval came through. Whilst this is a particular case, it does imply that tackling rare diseases is an area where Trump wants to see more speed from FDA. Getting both expedited approval and orphan designation would suggest that the drug is treating a priority area. So just how many expedited novel drug approvals were also orphans?
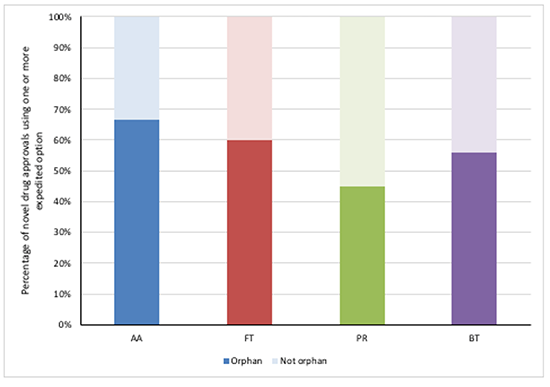
Between 2015 and 2017, more than half of novel drug approvals using Accelerated Approvals, Fast Track and Breakthrough Therapy designation were also orphan drugs and just below half were also given Priority Review (Figure 2).
Figure 2: FDA novel drug approvals between 2015 and 2017, using an expedited development and review method by orphan drug designation
Source: Analysis of FDA novel drug approvals data 2015, 2016 and 2017.
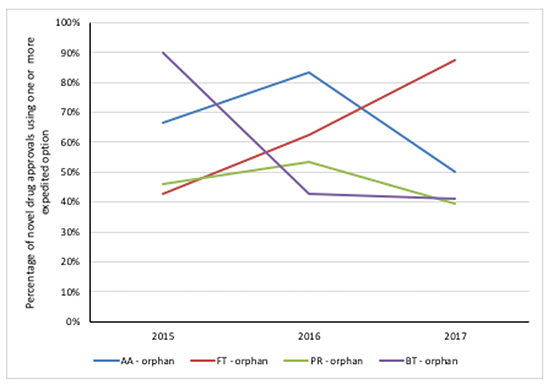
When the data is broken down for each year, it’s also clear that there has been changing trends across the different options for expedited development and review methods and Fast Track in particular has seen a rise in the overlap with orphan drug designation (Figure 3).
Figure 3: Trend in FDA novel drug approvals between 2015 and 2017, using an expedited development and review method by orphan drug designation
Source: Analysis of FDA novel drug approvals data 2015, 2016 and 2017.
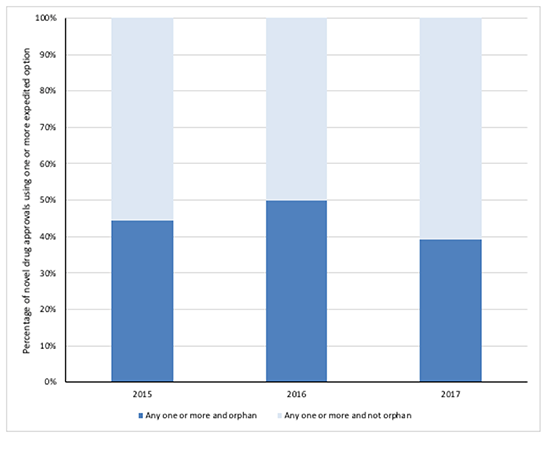
Whilst the detail behind each expedited review reveals a strong overlap, when looking at the novel drug approvals using one or more and orphan drug designation dilutes the findings (Figure 3). On this basis, the overlap never goes beyond 50 per cent. Arguably it’s more relevant to look at this picture because splitting out by the expedited review method abstracts from the reality of single drugs using a combination of the faster options at FDA.
Figure 4: FDA novel drug approvals between 2015 and 2017, using one or more expedited development and review method by orphan drug designation
Source: Analysis of FDA novel drug approvals data 2015, 2016 and 2017.
The right focus for speed?
If you take the views of the public and orphan designation as the right indicators, then the FDA faster approvals are tackling those but perhaps not at a level that you might expect. That leaves some unanswered questions, including why more orphans are not going through FDA fast lanes? Should orphans have not only incentives for the market-place, but also for speed of approval?
Leela Barham is a freelance health economist and policy expert. You can reach her on leels@btinternet.com. She is providing input as a subject matter expert into medicines pricing policy development with a UK government client and for the duration of her involvement in that project, she is restricted on what she can write about.
FDA Outlines Updated Requirement for Placebo-Controlled Trials in Vaccine Research
May 21st 2025In an article recently published by The New England Journal of Medicine, FDA higher-ups Vinay Prasad, MD, MPH; and Martin A. Makary, MD, MPH, wrote that any new COVID-19 vaccine must now be evaluated in placebo-controlled studies.
Navigating Distrust: Pharma in the Age of Social Media
February 18th 2025Ian Baer, Founder and CEO of Sooth, discusses how the growing distrust in social media will impact industry marketing strategies and the relationships between pharmaceutical companies and the patients they aim to serve. He also explains dark social, how to combat misinformation, closing the trust gap, and more.