
The obesity pipeline, despite a hugely underserved market potentially worth $11 billion, is awfully thin.

The obesity pipeline, despite a hugely underserved market potentially worth $11 billion, is awfully thin.
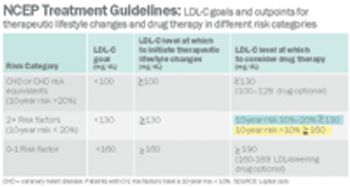
The world's largest drug manufacturer must answer off-label promotion charges brought by a new adversary. Not FDA, with its warning letters and threats of marketing sanctions, and not the Office of the Inspector General (OIG) at Health and Human Services, which often sues for fraud, forces huge settlements, and requires companies to do business under restrictive corporate integrity agreements. Instead, the company faces a class-action civil suit from insurance companies and union welfare funds, groups that, until recently, Pfizer regarded primarily as customers-or at least people who picked up the tab for customers. Now, led by the Welfare Fund of a Teamsters local from New Jersey, third-party payers are suing under RICO, the Racketeering Influenced and Corrupt Organizations Act. If their suit is successful, payers who have covered billions of dollars worth of Lipitor (atorvastatin) over the past five years will receive treble damages for the cost of off-label prescriptions. The suit may also attract..

The blockbuster era is at an end. Drugs are focused on smaller audiences today. With fewer doses to run, we must be flexible to support multiple molecules.

All of the companies with inhaled-insulin drugs must perform two-year safety studies. Not because their drugs show troubling data, but because Exubera has caused slight, temporary decreases in lung function.

Pharma has an enormous backlog of drugs that never made it out of clinical trials. At Gene Logic, these discontinued drugs spell opportunity. Not only are they the foundation of a new business unit at Gene Logic, such failed compounds may become the cornerstone of a new approach to R&D as the industry confronts its latest productivity crisis.

Published: September 1st 2009 | Updated: November 17th 2020

Published: April 1st 2006 | Updated: November 15th 2020

Published: February 1st 2006 | Updated: November 15th 2020
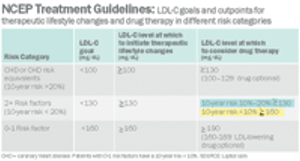
Published: May 1st 2006 | Updated: November 15th 2020

Published: March 1st 2006 | Updated: November 15th 2020