Pharmaceutical Executive
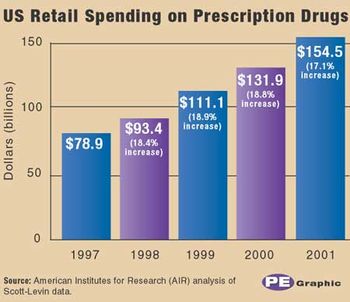
The National Institute of Health Care Management made headlines with its report on double-digit increases (17 percent) in retail spending on medicines in 2001. The total reached $155 billion last year, almost double the $80 billion spent in 1997, according to the study, "Another Year of Escalating Costs." PhRMA president Alan Holmer said the increase is a good thing, signifying that more people who need medicines for chronic conditions are being treated and thereby avoiding more expensive medical procedures.


