
Pharmaceutical Executive
BMS' use of investigational toxicology puts it in good stead with FDA, which, under its Critical Path initiative, is pushing for more complete toxicology packages.

Pharmaceutical Executive
BMS' use of investigational toxicology puts it in good stead with FDA, which, under its Critical Path initiative, is pushing for more complete toxicology packages.

Pharmaceutical Executive
Integra v. Merck KGaA supports research by large pharmaceutical companies, but it also opens the door to greater use of compounded materials by all parties. Congress may have to clarify its scope.

Pharmaceutical Executive
Imagine drugs that can detect one particular compound in a patient's body and respond to it by releasing a drug. They're not that far away.

Pharmaceutical Executive
Leadership evolves from the dynamic of particular situations. Without Adolf Hitler, Winston Churchill may have been remembered as a quirky backbencher.

Pharmaceutical Executive
SPCs extend a drug's basic patent protection for up to five years, to take into account the time that may have lapsed between the filing of a patent application and the granting of market authorization.
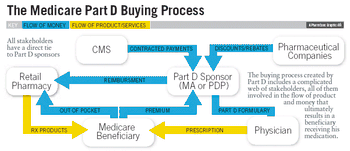
Pharmaceutical Executive
Manufacturers will have to incorporate Part D–specific messaging into all of their current marketing materials. In addition, they should consider publication plans and CME that specifically address the needs of seniors.

Pharmaceutical Executive
As a result of the rapid innovations in drug technology, and the increasing complexities surrounding drugs' safety, cost, and efficacy, the demand for extensive formulary reviews is growing. To keep pace, Pharmacy & Therapeutics (P&T) committees have been ardently reviewing medications to determine which ones deserve inclusion and preferred placements in health plans and formularies. While there are many factors that influence the committees' decisions, with some carrying more weight than others, pharmaceutical execs complain that there is no accurate way to predict which drugs will make the cut.

Pharmaceutical Executive
Elegant positioning strategies often fail when doctors learn that a prescribed product isn't on a patient's managed care formulary.