The 2008 Pipeline Report
We scrub industry's pipeline to find the drugs that everyone will be talking about in 2009 and beyond
This year's pipeline might be best remembered for drugs that evaporated at the last moment. Sanofi-Aventis gave up on Acomplia, one of the most celebrated drug candidates in the Pipeline Report for three years running, and took a whole crop of CB1 obesity drugs down with it. Flurizan, last year's headliner in Alzheimer's therapy, proved less than efficacious. And Prasugrel, once Eli Lilly's breathtaking balancing act of efficacy and safety, became the poster child for gridlock at FDA.
"It's grim," says Barbara Ryan, pharma analyst with Deutsche Bank. "There isn't a lot in late-stage development that we're really excited about."
But even in discouraging times, the academic, medical, and market experts who referee our drug candidates find compounds that promise therapeutic progress and even healthy profits. From the Factor Xa heart medications to new vaccines and cancer therapies, we take a look at 33 drugs that will matter in 2009 and beyond.
Asthma: chronic obstructive pulmonary disease (copd)
Paving the way for once-a-day
Novartis is poised to take a big piece of the market in chronic obstructive pulmonary disease (COPD) with its inhaled 24-hour bronchodilator indacaterol (QAB149), which will pave the way for new combination drugs that marry the new therapy with complementary compounds to create once-daily inhalants.

QMF149 will combine indacaterol with Asmanex (mometasone), a corticosteroid developed by Schering-Plough. The likely delivery device for once-daily dosage is S-P's Twisthaler, according to Adis R&D Insight.
Because the two long-acting compounds have different mechanisms of action, the combination therapy may result in greater efficacy.
"We see peak sales of $2.4 blllion in 2017 for QMF149," says Holger Rovini, a Datamonitor analyst in London. "GSK will no longer have the market to themselves." The drug's chief competitors are twice-daily inhalants, so the new drug is expected to have compliance advantages.
Rovini expects the second leading product in 2017 to be Novartis' long-acting single-dose inhalant QVA149 (indacaterol/glycopyrrolate), which will reach sales of nearly $1.9 billion worldwide. Datamonitor expects QVA149 to be particularly popular for the treatment of COPD patients.
Type 2 diabetes
Weekly dosing for GLP-1
In the treatment of type 2 diabetes, glucagon-like peptide-1 analogs—the class of drugs including Lilly/Amylin's Byetta (exenatide)—have a special place, according to Jay Skyler, MD, of the University of Miami's Miller School of Medicine. "GLP-1 therapy has the unique advantage of effective glucose control and concomitant weight loss," he says. "There appears also to be the potential for unique cardiovascular benefit." A crop of new once-a-week GLP-1 drugs promise to transform the class.
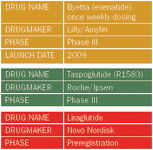
First up is a weekly formulation of Byetta, which as a twice-daily formulation remains a favorite among doctors and patients, despite persistent reports that it causes acute pancreatitis. A 30-week, open-label, non-inferiority Phase III study of 295 patients showed that once-weekly exenatide was more effective than twice-daily exenatide. But in early November, FDA rejected part of the data in the NDA having to do with equivalence, potentially delaying the drug's anticipated 2009 launch.
Taspoglutide, a human GLP-1 analog, has not been approved for daily use, and will begin its clinical life as a once- or twice-weekly treatment. Roche, which licensed rights to the drug everywhere but Japan and France, recently announced a Phase III study, triggering a €6.7 million milestone payment to Ipsen, which originated the compound and developed its excipient-free time-release mechanism. The study will enroll 990 patients with type 2 diabetes, and is scheduled to end in 2010.
The new drug liraglutide, from diabetes powerhouse Novo Nordisk, is being touted as the better Byetta. But to be approved, it first needs to satisfy FDA on pancreatitis. So says Les Funtleyder, healthcare strategist at Miller Tabak. FDA will see it as a class issue, even if Novo argues that diabetics just get more pancreatitis than the population as a whole. "If they have the pancreatitis data, fine," Funtleyder says. "If not, they'll have to get it."
Meningitis vaccines
A new Prevnar, plus new vaccines from Novartis and GSK
Established meningitis vaccines may lose market share to their successors beginning next year, when a fresh crop of multivalent vaccines is expected to hit the market.

Candidates by Wyeth and Novartis are expected to become at least $2 billion opportunities, according to Datamonitor's Holger Rovini.
Wyeth's Prevnar family of vaccines protect against pneumococcal serotypes that cause a variety of infections including blood infections and meningitis. The original Prevnar worked against seven serotypes. The new version, PNCRM13, also known as Prevnar 13v, targets those seven plus six more, including 19A, which is especially important in Asia. An additional indication is being sought for vaccinating the elderly. Rovini cited data that indicate vaccinating the very young can reduce the incidence of pneumococcal infections in the elderly, because vaccinated children do not infect their caregivers.
Synflorix, a 10-valent vaccine, excludes serotype 19A but adds non-typeable Haemophilus influenzae. The GSK drug could reach peak sales of $600–700 million.
Menveo could reap upwards of $2 billion for Novartis, according to Datamonitor. It competes with Sanofi-Pasteur's Menactra. Rovini notes that clinical data released by Novartis make Menveo appear superior, but the endpoints chosen may not prove any advantage over Menactra in terms of real-world benefits.
Pain
Breakthrough NSAIDS
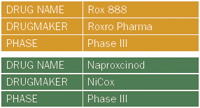
PIlls can be slow and relatively ineffective (to say nothing of addictive), and self-injection is a problem for many patients. As a result, too many post-operative patients spend extra days in the hospital. Rox 888, Roxro's new nasally delivered formulation of the NSAID keterolac solves both of those problems. Rox 888 is fast acting and has proven as effective as intramuscular injections of keterolac in the control of abdominal and orthopedic post-surgical pain.
"This is a drug that can be easily administered in a home environment," says Neil Singla, MD, of Lotus Clinical Research in Pasadena, CA. "That's a huge advantage."

Holger Rovini
Meanwhile NiCox, a French company with an attractive nitric oxide–contributing technology has received positive Phase III results on its naproxcinod—which hit all its end points without detrimental effects to patient blood pressure. At a time when chronic pain meds are increasingly under fire for cardiac side effects, naproxcinod and the class of drugs coming up behind it could become major players.
Autoimmune disorders
New targets, small molecules, oral delivery
An orally active immunosuppressant, Pfizer's CP690550 could be the long-sought small-molecule treatment for rheumatoid arthritis, says Clare Davies, lead analyst for immunology and inflammation at Datamonitor. An RA pill would help payers and patients alike, since monoclonal antibodies are all injectibles. The new drug attacks lymphocytes by inhibiting the Janus-activated kinase 3 (JAK3) pathway. In addition to RA, the therapy is being tested for Crohn's disease.

Another promising small molecule inhibits the related JAK2 enzyme. Incyte's INCB018424 not only promises efficacy against rheumatoid arthritis but is also in trials to treat multiple myeloma, prostate cancer, and psoriasis. Once again, the fact that the drug is an orally available daily-dose treatment should encourage patient compliance.
ChemoCentryx and GlaxoSmithKline are partners in the discovery, development, and marketing of compounds targeting chemokine and chemoattractant receptors for the treatment of inflammatory disorders. Two of these compounds aim to treat various forms of inflammatory bowel disease, including Crohn's disease, celiac disease, and ulcerative colitis. Their mechanism: selective binding to the CCR9 chemokine receptor, which according to the company is a "highly specific receptor expressed by T cells that migrate selectively to the digestive tract."
Currently in a Phase II/III trial for Crohn's disease is the first of these compounds, Traficet-EN (CCX282-B). Behind it, in Phase I, is CCX025. GSK paid $63.5 million in upfront cash and equity investments for world marketing rights to Traficet-EN, CCX025, and two other compounds. Milestones payments could reach $1.5 billion.
DIABETES: INHALED INSULIN
A breath of fresh air?
After Pfizer withdrew Exubera from the market in October 2007, the once-crowded field of inhaled insulin candidates thinned to a single product: MannKind's Afresa. But instead of convenience, the product—still better known as Technosphere insulin—appears to be surviving on clinical benefit.
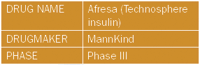
"I am still optimistic that MannKind's Afresa will be sufficiently beneficial because of its profile to be successful in the market even though Exubera failed," says Jay Skyler.
MannKind's unique technology allows an acidic crystalline powder dusted with insulin to liquify at neutral pH deep inside the lungs and seep into the bloodstream across tiny pulmonary membranes. The relatively quick delivery of insulin (slower than the pancreas, but faster than subcutaneous injections) creates an insulin profile more like the body's own than any other drug available, according to Skyler. In addition, the drug disappears faster from the bloodstream when its work is done.
In September, MannKind released top-line results of a Phase III clinical trial comparing Afresa to subcutaneous insulin aspart. The inhaled product better prevented incidents of hypoglycemia and glucose spikes after meals. It had no pulmonary side effects, and it had a welcome benefit for diabetes sufferers: an average weight loss of 4.4 pounds in 52 weeks for Afresa users with type 1 diabetes, compared to a three-pound weight gain for patients on insulin aspart.
The drug may have to carry a class-wide warning for cancer, but company market research suggests that even with a tough label, Afresa is a potential blockbuster.
Oncology
New agents for prostate, renal, and basal cell cancer
There are a record 750 oncology drugs in pharma's pipeline. Many won't make it to market—and even if they do, it will be easier than ever for some to get lost in the shuffle. Here are some cancer drugs we think will be impossible to ignore:

First off is Cougar Biotechnology's abiraterone (CB7630), a prostate cancer pill. Some studies have shown that prostate tumors grow by producing their own testosterone, and abiraterone attacks this mechanism by inhibiting CYP17A1, an enzyme involved in the production of testosterone. Fleur Pijpers, Datamonitor's oncology analyst. Pijpers forecasts that sales for this second-line hormone-refractory prostate therapy will reach $515 million by 2017, excluding whatever earnings Cougar gains from other therapies using the same molecule.
Axitinib (AG13736) is Pfizer's follow-on to Sutent (sunitinib), its receptor tyrosine kinase (RTK) inhibitor. The drug inhibits multiple targets, including VEGF-1, and is being tested for pancreatic, thyroid, and renal cancer. "This could be the next big thing," says Pijpers. "We have to wait for the Phase III results to come out, but for now it looks pretty decent—and with Pfizer's experience in renal cell carcinoma, it could do quite well." The drug targets slightly different kinases than Sutent—and has a slightly different MOA—which is why it might be a good follow-on to the earlier product, Pijpers says. It is regarded as a monotherapy, at least in renal cell carcinoma.
Another promising antiangiogenic is pazopanib, GSK's VEGF-2 inhibitor. Pazopanib also targets platelet-derived growth factor receptor (PDGFR) and c-kit. Like its cousin Avastin, the drug could also work for age-related macular degeneration.
"It's a promising drug," says Bruce Chabner, MD, clinical director at Massachusetts General Hospital in Boston. "It's active against lung cancer, sarcomas, and renal cell cancer."
Novartis' Afinitor (everolimus) is an MTOR inhibitor similar to Wyeth's sirolimus. Both have been launched as anti-rejection drugs after kidney transplants (Novartis' under the brand name Certican, Wyeth's as Rapamune). Afinitor is in trials for a number of cancers and is awaiting approval for advanced kidney cancer. (At press time, the company announced that FDA had requested additional data on the drug and that approval would be delayed by several months.)
Elesclomol is an injectable apoptosis stimulator aimed at melanoma, which is one of the tumor types for which the fewest treatment options exist. Old systemic chemotherapies are still used for metastatic patients, although they are generally thought to be ineffective, and off-label prescriptions are in use as well, according to Pijper.
"Drug developers are interested in tumor types like melanoma," says Pijper about the Synta/GSK project, "Even though their incidence is not as high as the big four tumor types: breast, prostate, colorectal, and lung cancer. The unmet needs of melanoma are so great that if a drug gets approved, it has a good chance of being commercially successful."
Ramcirumab is a fully human monoclonal antibody that could have fewer side-effects than Avastin, a humanized monoclonal antibody. Lilly scooped up ImClone in part due to this drug—but expect an uphill battle for the developer. The Phase III trial won't end until 2012. By that time, Avastin may so dominate the market that it could prove impossible for ramcirumab to gain a toehold. "But if the drug shows simply amazing results," Pijpers says, "It might do all right."
Further back in the pipeline, GDC-0449 by Curis and Genentech is creating a stir with its new MOA against basal cell cancers. The drug interferes with the so-called hedgehog pathway, which has a mutation in the basal cell and colorectal cancers. The drug has a long way to go, "but it was very very active against basal cell cancers and that is important," Chabner says.
Finally, one of the most talked about classes of new cancer drugs—PI3-k inhibitors—has made it to the clinic. "There is a mountain of evidence that implicates the PI3-K pathway in everything bad in cancer from A to Z: growing, metastasizing, invading, causing growth of blood vessels, resisting cell death," said Chabner. It's too early to say much about them as treatments, but watch for these therapeutics in future Pipeline Reports.
Migraine
An alternative to triptans—the first in 20 Years
Merck's Maxalt (rizatriptan) is the current US standard of care for migraines. But the company will capture an even bigger piece of the acute migraine market if its telcagepant (MK0974) is approved. An antagonist of the calcitonin gene-related peptide (CGRP) receptor, it stands to be the first approval in a new class of migraine drugs in nearly 20 years—since the first tryptamine (or triptan) drug was approved in 1991.

The drug offers an alternative, but still needs to prove itself in the real world. "It looks like Merck's new drug lacks the cardiovascular toxicities of the triptans," says Barbara Ryan, pharma analyst at Deutsche Bank. "It doesn't look like there is anything dramatic to report in the efficacy department. It's just a question of how much better it will be than triptans, and what kind of market share it will get in the current climate when everyone is focused on costs and the triptans are going generic."
A Phase III study published in the Lancet in November compared telcagepant with AstraZeneca's triptan, Zomig (zolmitriptan). In the study telcagepant showed effectiveness similar to zolmitriptan's but with fewer side effects. An accompanying editorial said the result "marks a new era in migraine therapy."
CGRP receptor antagonists inhibit the transmission of pain signals in the brainstem by blocking the release of CGRP. Merck is developing other CGRP antagonists as back-up compounds to telcagepant. "There is clearly a market here," Ryan says. "The new drug may work for the people who don't respond or can't tolerate the triptans."
Merck expects to file an NDA in this indication in 2009.
Acute coronary syndrome/stroke
The Generation X of anticoagulants
Two new factor Xa inhibitors with potent anticoagulant properties are leading candidates to make an impact on heart-attack and stroke prevention.
"This is a big market with a huge unmet need, and these are oral therapies," says Barbara Ryan. "So there is a lot of room for efficacious drugs. The question is how efficacious and safe are they going to be?"
Factor Xa is a catalyst for the proteolysis reaction that converts prothrombin to thrombin and stimulates blood clotting. Inhibiting this reaction stops harmful blood clots. It is thought that Factor Xa drugs will require less monitoring than traditional anticoagulants and thus will be useful in indications such as atrial fibrillation.
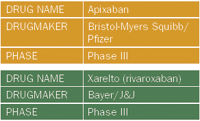
Both apixaban (from BMS and Pfizer) and Xarelto (rivaroxaban, from Bayer and J&J), are in Phase III trials for treatment of deep vein thrombosis (DVT) and pulmonary embolism, which is often a consequence of DVT. BMS is testing apixaban head-to-head against enoxaparin/warfarin, the current standard of care for DVT in the United States. Xarelto has been approved in Canada and Europe for prevention of post-surgical venous thromboembolism in patients undergoing elective hip or knee-replacement surgery, and it is currently in registration in the United States for a similar indication.
But the potentially big payoff for both drugs turns on the results of the Phase III acute coronary syndrome (ACS) and stroke-prevention trials. "The problem with these cardiovascular indications like ACS and stroke is that it all comes down to the outcome studies," Ryan says. "Before that you are just looking at markers and it's hard to say."
Alzheimer's disease
Getting new drugs into the brain
Since June, when Encore/Myriad's Flurizan failed to achieve its efficacy endpoints in Phase III, experts have been divided on the future of Alzheimer's drugs in the pipeline. A key question: Will the beta-amyloid pathway turn out to be the right target?

But Jeffrey Cummings, MD, director of the Alzheimer's Disease Center at UCLA, sees the issue as challenging technical problems rather than a problem with the approach. "What we learned from the Flurizan trials was that the drug did not enter the brain adequately to effect a response," says Cummings.
Cummings said that recent research suggests that lipid-soluble drugs are more likely than water-soluble chemicals to enter the brain, and that the three current Phase III Alzheimer's candidates are more likely to be found in concentrations large enough to actually attack the beta-amyloid plaque that, it is widely believed, causes the dementia suffered by Alzheimer's patients.
"In the development program, they have actually been measured in the spinal fluid to make sure that they are entering the brain," Cummings said.
Indeed, Cummings remains cautiously optimistic about Elan/Wyeth's monoclonal antibody bapineuzumab and Lilly's gamma-secretase inhibitor semagacestat. For him, the real question is about the point of intervention. Amyloid has been detected in the brains of people five to ten years before the onset of symptoms. Some of these have been followed long enough to confirm the hypothesis that they are the ones who will get Alzheimer's.
"We have amyloid scans that show the presence of amyloid in the brain of people who are normal, or at most mildly impaired," Cummings says. "One possibility is that treating patients as we choose them now—by the presence of the dementia syndrome—may already be too late for the optimal intervention with drugs that are aimed at amyloid."
"The drug that is currently in the lead to become the next treatment of Alzheimer's disease is called Dimebon," Cummings says. Dimebon (dimebolin) originated in Russia in the 1980s as an oral antihistamine. Medivation, which began development for neurodegenerative disorders, licensed the drug to Pfizer in September. Dimebon appears to affect the beta-amyloid pathway, although the precise mechanism of action is not known. The drug did well in a US-run Phase II trial with cognitively impaired patients in Russia. "It appears to be a neuro-protective agent that could work much later in the disease when people are symptomatic," Cummings says.
CTS 21166 is a beta-secretase inhibitor that aims to block the beta-amyloid pathway at a new point of attack. In early Phase II, it is one of the most promising drugs further up the pipeline, Cummings says.
Multiple sclerosis
First oral drug for a deadly disease
This is a new orally available drug for one of the most poorly treated afflictions in neurology. "If there were ever a disease with an urgent unmet medical need, it is MS," says Les Funtleyder, health care strategist at Miller Tabak.
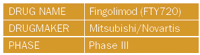
Originally derived from a fungus, Isaria sinclairii, fingolimod (FTY720) uses a new mechanism of action involving a so-called S1PR agonist to bind selectively to circulating lymphocytes and keep them from leaving the lymph nodes. This results in fewer activated T-cells in the bloodstream and central nervous system.
"All of the other MS drugs are injectibles, so the fact that it's an oral therapy is a big plus," says Funtleyder, noting that many treatments, including interferons, also have debilitating side effects. "If it is approved, just proving non-inferiority to other treatments will enable it to do well commercially."
The current Phase III trial is scheduled to end in 2013.
Osteoporosis/bone cancer
Will denosumab get a break?
A fully human monoclonal antibody that prevents the RANK ligand from binding to its receptor, denosumab inhibits the differentiation of osteoclasts, which break down bone, thus preventing the bone resorption associated with osteoporosis. The drug is likely to win approval. The question is whether payers will spring for a premium over traditional therapy—and especially the bisphosphonates, some of which are available as generics. Denosumab is a once or twice-yearly injection, which has obvious compliance advantages. But the challenge will be to get payers to spring for a premium over bisphosphonates.

Amgen released Phase III datra this fall showing that denosumab outperformed Fosamax. Will it be enough? Or will Amgen have to wait until the drug picks up a cancer indication to realize its potential?
Antiplatelets
Still waiting for prasugel
If ever a drug seemed to inspire FDA to sit on its hands, Effient (prasugrel) is it. An outside observer might be forgiven for assuming that FDA has most to gain by indecision.

Prasugrel promises to be a more efficacious alternative to Plavix, which will go generic in 2011, and to pose uncalculated dangers, if doctors try to use it in populations too prone to bleeding. The potential for adverse effects, even with a strongly worded warning, is high. And the drug will be pricey for payers.

Les Funtleyder
"The issue for FDA is whether prasugrel is meaningfully better than Plavix," says Les Funtleyder. "All the recent drug withdrawals have made them much more gun shy."
The drug may make it. "But with two FDA delays, its chances are not good," Funtleyder says.
Addressing Disparities in Psoriasis Trials: Takeda's Strategies for Inclusivity in Clinical Research
April 14th 2025LaShell Robinson, Head of Global Feasibility and Trial Equity at Takeda, speaks about the company's strategies to engage patients in underrepresented populations in its phase III psoriasis trials.
The Misinformation Maze: Navigating Public Health in the Digital Age
March 11th 2025Jennifer Butler, chief commercial officer of Pleio, discusses misinformation's threat to public health, where patients are turning for trustworthy health information, the industry's pivot to peer-to-patient strategies to educate patients, and more.
