- Sustainability
- DE&I
- Pandemic
- Finance
- Legal
- Technology
- Regulatory
- Global
- Pricing
- Strategy
- R&D/Clinical Trials
- Opinion
- Executive Roundtable
- Sales & Marketing
- Executive Profiles
- Leadership
- Market Access
- Patient Engagement
- Supply Chain
- Industry Trends
AstraZeneca Acquires Amolyt Pharma with the Aim of Developing Rare Endocrine Disease Treatments
Acquisition is highlighted by eneboparatide, Amolyt’s Phase III peptide for hypoparathyroidism with a novel mechanism of action.
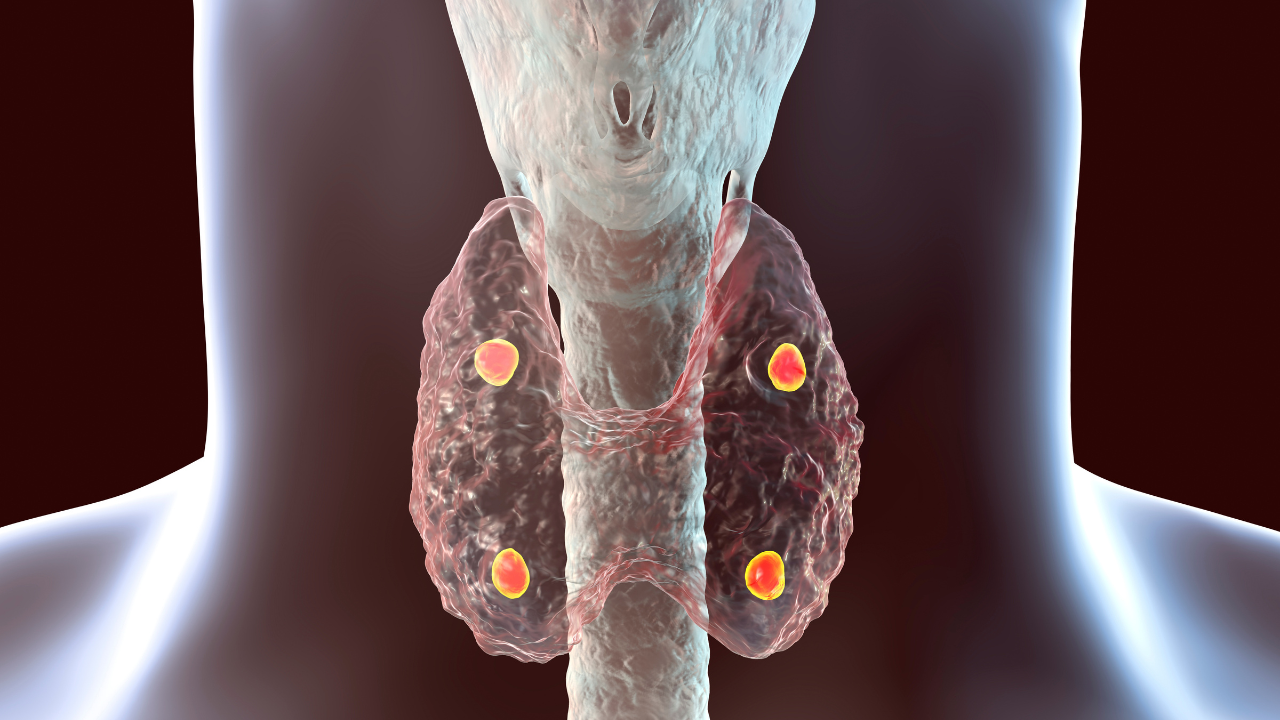
AstraZeneca announced that it has acquired Amolyt Pharma, a biotech company focused on developing novel treatments for rare endocrine diseases. Through this acquisition, AstraZeneca said it hopes to expand on the Alexion, AstraZeneca Rare Disease subsidiary pipeline through the addition of eneboparatide (AZP-3601), Amolyt’s novel peptide for hypoparathyroidism. Currently, the treatment is in Phase III of clinical trials.1
“Chronic hypoparathyroid patients face a significant need for an alternative to current supportive therapies, which do not address the underlying hormone deficiency. As leaders in rare disease, Alexion is uniquely positioned to drive the late-stage development and global commercialization of eneboparatide, which has the potential to lessen the often-debilitating impact of low parathyroid hormone and avoid the risks of high-dose calcium supplementation,” said Mark Dunoyer, CEO, Alexion, AstraZeneca Rare Disease, in a press release. “We believe this programme, together with Amolyt’s talented team, expertise and earlier pipeline, will enable our expansion into rare endocrinology.”
Amid constructive discussions with the FDA, the Calypso Phase III clinical trial for AZP-3601 began last May after topline data from Phase II showed positive results. Participants from the US, Canada, Europe, and the UK were set to participate at 50 different sites. The Phase II trial found that eneboparatide maintained mean serum calcium within the target range, allowing discontinuation of oral supplements in 93% of patients.2
“We appreciate the constructive discussions with the FDA during the End-of-Phase 2 meeting and have designed our trial in line with the Agency’s guidance. We believe the Calypso study will be the largest prospective randomized clinical trial in hypoparathyroidism to date, representing a major milestone for the patient community as well as for Amolyt Pharma,” said Thierry Abribat, PhD, founder, CEO, Amolyt Pharma, in an earlier press release. “We look forward to further evaluating the clinical potential of eneboparatide to uniquely address key treatment goals in hypoparathyroidism. On the heels of our recently announced $138M Series C financing, we look forward to diligently executing this trial with the aim to announce topline data by the end of 2024.”
According to Medscape, hypoparathyroidismis a parathyroid hormone (PTH) deficiency. Some symptoms of hypoparathyroidism include, but are not limited to:
- Muscle cramps in the back and lower legs
- Mental health issues such as anxiety, depression, and psychosis
- Dry and coarse skin
- Spastic paraplegia, ataxia, dysphagia, and dysarthria3
“Patients undergoing parathyroidectomy for parathyroid hyperplasia are at high risk of developing permanent primary hypoparathyroidism. Patients may be treated with an autotransplant of a segment of parathyroid gland to prevent hypoparathyroidism,” said Joseph Michael Gonzalez-Campoy, MD, PhD, FACE, medical director, CEO, Minnesota Center for Obesity, Metatbolism, and Endocrinology, in a Medscape article on hypoparathyroidism statistics. “This autotransplant is usually placed subcutaneously in the forearm or in the neck. If the autotransplantation fails, patients receive the same treatment that is administered to other patients with hypoparathyroidism.”
Under terms of the agreement, AstraZeneca will pay a total of $1.05 billion for all outstanding shares, including $800 million upon closing and an additional $250 million upon the completion of specific milestones. The deal is expected to close later this year.1
“We enthusiastically welcome the proposed acquisition of Amolyt by AstraZeneca, an organization that shares our dedication to delivering life-changing treatments to people living with rare diseases,” said Abribat. “This agreement offers the opportunity to meaningfully advance our pipeline therapies. Strong Phase II data suggest eneboparatide has the potential to improve outcomes for patients and to shift the treatment paradigm for hypoparathyroidism, and we look forward to seeing the continued advancement of the Phase III trial.”
References
1. AstraZeneca to acquire Amolyt Pharma, expanding late-stage rare disease pipeline. AstraZeneca. March 14, 2023. Accessed March 14, 2023. https://www.astrazeneca.com/media-centre/press-releases/2024/astrazeneca-to-acquire-amolyt.html
2. Amolyt Pharma Announces Phase 3 Clinical Trial of Eneboparatide for the Treatment of Hypoparathyroidism following Positive End of Phase 2 Meeting with FDA. Amolyt Pharma. May 2, 2023. Accessed March 14, 2024. https://amolytpharma.com/2023/05/02/amolyt-pharma-announces-phase-3-clinical-trial-of-eneboparatide-for-the-treatment-of-hypoparathyroidism-following-positive-end-of-phase-2-meeting-with-fda/
3. Hypoparathyroidism. Medscape. October 10, 2022. Accessed March 14, 2024. https://emedicine.medscape.com/article/122207-overview#:~:text=Hypoparathyroidism%20has%20an%20estimated%20prevalence,22%20per%20100%2C000%20person%2Dyears.
Cell and Gene Therapy Check-in 2024
January 18th 2024Fran Gregory, VP of Emerging Therapies, Cardinal Health discusses her career, how both CAR-T therapies and personalization have been gaining momentum and what kind of progress we expect to see from them, some of the biggest hurdles facing their section of the industry, the importance of patient advocacy and so much more.
