The Challenge of Forecasting Biosimilars
The biosimilars market forecaster must create a framework that captures the dynamics that operate in these markets and do justice to the uncertainty and quantification of assumptions, writes Arthur G. Cook
The introduction of biologic medicines revolutionized treatment in several key therapeutic areas, including oncology, diabetes and various inflammatory diseases. The landscape for biologics will change dramatically in the next decade as many products – more than $60 billion in worldwide sales – lose their market exclusivity. In response, many companies are striving to bring similar biologic products, or “biosimilars,” to the market to capitalize on the innovator’s loss of exclusivity.
The biosimilars market generates many challenges for the forecaster, who must create a framework that captures the dynamics that operate in these markets – and do justice to the uncertainty and quantification of assumptions.
The Forecast Framework
A comprehensive forecast framework for biosimilars must take into account a number of uncertainties in today’s environment that affect both the forecast construct and assumptions. Specifically:
- Regulatory uncertainties regarding extrapolation and interchangeability;
- Legal uncertainties around intellectual property protection and the possibility of “at risk” launches; and
- Market uncertainties that range from provider and patient acceptance of biosimilars, to the mechanisms payers may introduce to drive the market toward the (presumably lower cost) biosimilar.
Regulatory Uncertainties
The FDA has not issued guidance on extrapolation for biosimilar products. Extrapolation refers to the usage of biosimilars for medical conditions or in patient populations that have been approved for the reference (brand name) biological – but for which the biosimilar has not been investigated. Extrapolation of indications would significantly reduce the volume (and therefore burden) of clinical trials that biosimilar producers need to undertake.
Arguments against extrapolation posit that a variation in the biosimilar molecule may not affect efficacy for one indication, but could affect the molecule’s efficacy for another indication. European guidelines allow for extrapolation, whereas Canadian guidelines do not.
For example, Sandoz developed a biosimilar of Amgen’s Neupogen (filgrastim), and obtained regulatory approval from the FDA in January 2015. In its decision, the FDA granted extrapolation for the biosimilar, referencing significant post-launch experience of the biosimilar in Europe. However, the FDA did not issue guidance on extrapolation for other biosimilar applications.
Interchangeability refers to the substitution of the branded product by a biosimilar without the intervention of the prescriber. If interchangeability is granted for a biosimilar, it may be substituted for the originally prescribed product at the pharmacy. This decision is determined at the state level in the U.S., whereas it is determined at the national level in Europe.
What does this mean for the forecaster? If extrapolation is granted, the biosimilar may access multiple therapy areas with a single regulatory approval. This argues for scenario forecasting, where the forecaster constructs discrete scenarios aligned with the possible regulatory outcomes. Having scenario forecasts available once the extrapolation decision is made enables a company to react quickly.
Interchangeability appears to be an unlikely event, at least in the short-term until the market has more experience with biosimilars. For this uncertainty, the forecaster would note it as “a risk to the forecast,” but would not move forward with forecast scenarios at this point.
Legal Uncertainties
Uncertainties around intellectual property protection arise due to the complexity of patents that govern the manufacture of biologics. Even after a biologic loses its active ingredient patent protection, patents still likely protect parts of the drug manufacturing process. In the U.S., the Biologics Price Competition and Innovation Act authorizes biosimilar registration and provides a period of time when the biosimilar producer shares their molecule production process with the original producer. The innovator will then provide the biosimilar manufacturer with a list of potential patent infringements. The intent is that the two parties will come to an agreement on how the biosimilar producer will provide compensation for the violated patents. This process is referred to as the “patent dance.”
However, a recent court decision has raised uncertainty around adherence of the patent dance. Sandoz did not engage in the patent dance with Amgen when it decided to make a biosimilar of Neupogen (filgrastim), because it claimed that it was an optional process. While Amgen filed for an injunction against Sandoz, the U.S. District Court in March 2015 agreed with Sandoz that sharing the information was optional. Amgen has since appealed the decision.
Interpreting sections of the law as “optional” favors biosimilar manufacturers, because it potentially allows them access to the market before all patent issues are settled. A biosimilar producer may choose to launch their product before the resolution of the litigation surrounding the patents used to produce the molecule. If the courts later favor the original producer regarding the patents in question, the biosimilar producer could be responsible for paying damages to the original biologic producer. However, the biosimilar producer may decide to accept this risk, given the value of obtaining the advantage of being the first biosimilar for a particular drug.
Since 2011, a biosimilar manufacturer in the U.S. also may attempt to invalidate patents using challenges through the U.S. Patent and Trademark Office rather than the court system. These challenges are done through an inter partes review filing, which petitions the Patent Office to invalidate a specific patent.
What does this mean for the forecaster? The possibility of “at risk” launches brings uncertainty to the launch date the forecaster must assume for a biosimilar product. As with regulatory uncertainty, the forecaster constructs a series of scenarios that examine the effects of at risk launches at varying approval dates. This process requires close communication between a firm’s legal team and forecast team.
Market Uncertainties
There are a number of stakeholders in the market who can influence the use of biosimilar products –health care providers, payers, patients and pharmacists – all of whom pose questions the forecaster must address. To what extent do health care providers adhere to their experience with the innovator biologic versus switching to prescribing a biosimilar? Payers seemed positioned to drive usage for the (presumably lower priced) biosimilar, but what mechanisms will they employ? Will a difference in patient copayment levels affect product use? What role will pharmacists play?
As with regulatory and legal uncertainties, the forecaster assesses these dynamics using scenarios. However, here the scenario constructs are more complex due to the number of stakeholders in the market. We approach the market uncertainties using the example construct shown in Figure 1.
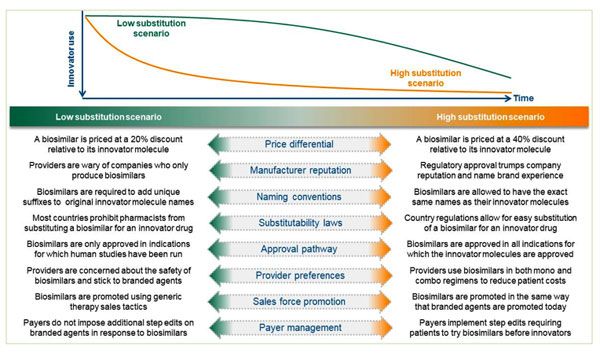
The actual construct employed reflects the specific therapy area to be forecasted. In the above example, the forecaster would place each assumption between the end points of the scales to create the scenario to be forecasted. If the assumptions align with a “high substitution” scenario, the forecaster would use a steep erosion curve for the innovator product (or, analogously, a steep adoption curve for the biosimilar). The converse would be true for assumptions aligned with a “low substitution” scenario.
The forecaster would determine each assumption by using secondary data, primary market research, analog products and inputs from each functional area within the organization.
As we discussed, the uniqueness of biosimilar forecasting arises from the broad uncertainties surrounding the forecast. Deriving the “most likely” assumptions in forecasting is always challenging; doing so in an environment fraught with regulatory, legal and market uncertainties that create additional complexity. The only sound approach to forecasting biosimilars relies upon clear articulation of the logic construct used in each scenario. In this way, the forecaster provides the key decision makers with both an assessment of performance for the biosimilar, as well as insight into the risk inherent in the forecast construct.
About the Author

Arthur G. Cook is a principal at global sales and marketing consultancy ZS Associates. He is the author of Forecasting for the Pharmaceutical Industry.
The Misinformation Maze: Navigating Public Health in the Digital Age
March 11th 2025Jennifer Butler, chief commercial officer of Pleio, discusses misinformation's threat to public health, where patients are turning for trustworthy health information, the industry's pivot to peer-to-patient strategies to educate patients, and more.