Compliance Regulations in the Trump Era
We know deregulation is coming, although we do not know what it will look like. The one looming question for pharmaceutical and medical device companies is about the future of compliance and regulations.
Change is afoot. No one knows exactly what it will look or feel like, but we know deregulation is coming. In the United States, we already have some insight into the future. The market’s initial upward trend in the wake of Donald Trump’s election victory points to the hopes corporate America has pinned on the presidency of the first businessman to hold the office. There is a belief in a coming renaissance because Trump himself promised to help boost revenues across the board, put American workers and companies first, cut corporate taxes, and deregulate.
The one looming question, especially for pharmaceutical and medical device companies, is about the future of compliance and regulations. What’s important to know for life sciences companies is that the DOJ upholds the laws through investigations of violations of The Foreign Corrupt Practices Act of 1977 (FCPA), Anti-kickback Statute, and the False Claims Act just to name a few. Looking at who is coming and going in these offices sheds light on what the life sciences industry can expect.
In the meantime, cases and settlements continue to pop up. The life sciences industry has seen an increase in DOJ investigations into corrupt business practices within the pharmaceutical and medical device sector since 2005. On the heels of the Trump Inauguration, Teva Pharmaceutical’s announced a $519 million selttlement for violation of the FCPA in Ukraine, Mexico, and Russia. And ZimmerBiomet’s repeat FCPA violations settlement of $30 million for illegal conduct in Brazil and Mexico was announced on Jan. 12, 2017. There are many others (see graphic).
At the same time, the SEC is making its own changes. Leslie R. Caldwell, who specializes in prosecuting white-collar cases, tendered her resignation Jan. 12. This departure came after Mary Jo White, the thirty-first chair of the SEC, became the first major appointee of former President Barack Obama to resign after Trump’s victory, indicating that she might think her hardline on prosecuting violators would no longer be a priority with the new president. With these departures at the SEC, the Trump White House has the ability to change and shape the “tone from the top” in an unprecedented manner.
Still, the laws will remain intact for the time being even if they are not President Trump’s priority, and committing violations leaves companies vulnerable to prosecution, which is never good for the bottom line. Uncertainty is the only certainty at this point. That’s why having a strong compliance strategy in place is the safest bet for all life sciences companies.
Other notable FCPA enforcements include:
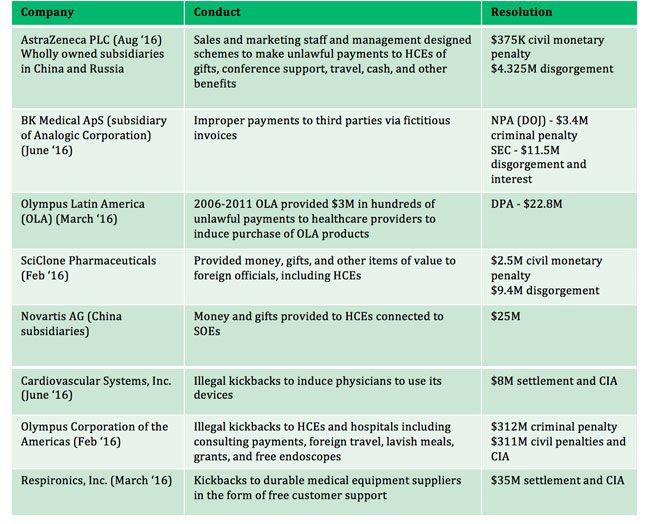
Source: “MediSpend-Navigant Global Compliance Webinar: New Standards for Enterprise Compliance for the Life sciences Industry” November 7, 2016 webinar.
Despite the uncertainty, here’s what to do about managing compliance in your organization:
• Live by the Law
What is certain is the fact that anti-corruption and global transparency laws are still on the books and will be for the foreseeable future. Failing to follow the regulations puts your company at risk of having to pay hefty fines, face devaluation of your company due to bad publicity, and losing trust of physicians and patients in the long run. Businesses have no way of knowing if enforcement from U.S. agencies will get more relaxed in the months or years to come. So do the right thing. Run an ethical and compliant organization.
• Set the “Tone from the Top”
Compliance and transparency is good for business if the tone is set from the top and permeates throughout the company culture with employees, customers and investors. That only happens when the CEO, Board of Directors, and most senior of executives of the company set in place a corporate culture of transparency and represent through their words and actions a culture of compliance. All employees must embrace ethical behaviors and senior leaders and middle managers must serve as role models to ensure the message is heard and carried throughout the organization. Leaders must demonstrate the behavior they expect employees to emulate, train employees on compliant behavior and anti-corruption policies, and demonstrate it in their daily behavior. In other words, be ethical to promote ethics.
• Institute an Effective Compliance Program
Implementing and maintaining an effective compliance program first starts with understanding the current culture of the business and taking an honest look at accountability. A good risk-based compliance program should include seven essential elements: 1) Oversight and Governance, 2) Written Standards, 3) Communication, 4) Training, 5) Auditing and Monitoring, 6) Investigations, and 7) Corrective Action.
• Analytics to Manage Risk
A new element, called the Eighth Element of a good compliance program is a Risk Assessment and Management Program (RAMP). Implement a cloud-based compliance software solution that provides a standardized, centralized risk assessment and mitigation process to track, evaluate, and identify risks associated with its business functions. The RAMP workflow includes control points to monitor business processes and should engage key business stakeholders such as compliance, legal, and business unit leaders, engaged in an end-to-end automated workflow that allows monitoring and control points throughout the entire healthcare provider (HCP) engagement process. Evalution of business practices to identify risks, gaps and bad actors in the company should become part of the daily activities and responsibilities of the compliance, legal, and business teams.
Taking these steps will ensure that no matter what the future holds, your company will be ready.
Michaeline Daboul is the CEO and co-founder of MMIS | MediSpend. She introduced MediSpend, the first open source Software-as-a-Service (SaaS), compliance cloud solution to the life sciences industry in 2011. Contact Michaeline at mdaboul@medispend.com and follow her on Twitter @mmispresident. LinkedIn: https://www.linkedin.com/in/michaelinedaboul
FDA Approves AbbVie’s Rinvoq as First Oral JAK Inhibitor for Giant Cell Arteritis
April 30th 2025AbbVie secures FDA approval for Rinvoq as the first oral Janus kinase inhibitor indicated for giant cell arteritis, expanding its immunology portfolio and signaling strategic growth opportunities in underserved autoimmune markets.
Navigating Distrust: Pharma in the Age of Social Media
February 18th 2025Ian Baer, Founder and CEO of Sooth, discusses how the growing distrust in social media will impact industry marketing strategies and the relationships between pharmaceutical companies and the patients they aim to serve. He also explains dark social, how to combat misinformation, closing the trust gap, and more.