
Clarivate’s Drugs to Watch 2026 report highlights Eli Lilly’s late-stage cardiometabolic candidates orforglipron and retatrutide as the clear standouts in an obesity and diabetes market projected to reach $150 billion by 2035.

Clarivate’s Drugs to Watch 2026 report highlights Eli Lilly’s late-stage cardiometabolic candidates orforglipron and retatrutide as the clear standouts in an obesity and diabetes market projected to reach $150 billion by 2035.
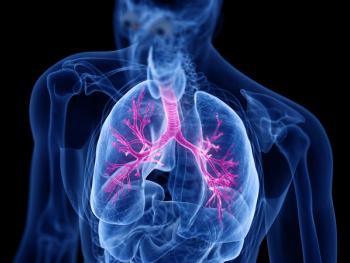
The approval is supported by Phase III SWIFT-1 and SWIFT-2 trial data showing that twice-yearly dosing of Exdensur significantly reduced annualized asthma exacerbation rates compared with placebo when added to standard of care.
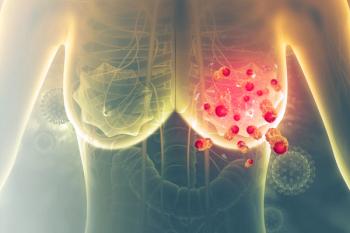
The approval is based on Phase III DESTINY-Breast09 data showing the Enhertu–Perjeta combination significantly improved progression-free survival compared with the current taxane-based standard of care in previously untreated metastatic disease.

The 16th addition to the FDA Commissioner’s National Priority Voucher pilot program follows Phase III MajesTEC-3 trial data showing significant survival benefits for the Tecvayli-based regimen compared with standard of care in treating relapsed or refractory multiple myeloma.

The FDA’s approval of Uplizna for antibody-positive generalized myasthenia gravis introduces a twice-yearly CD19-targeted therapy option for the rare autoimmune condition.
Epkinly plus rituximab and lenalidomide is the first bispecific antibody combination FDA-approved for relapsed or refractory follicular lymphoma, backed by Phase III data showing substantially improved disease control over standard therapy.

Approval of Komzifti (ziftomenib) provides a new targeted option for a high-risk patient population and strengthens the Kura Oncology–Kyowa Kirin collaboration, which includes a global development and commercialization strategy.

The FDA has added six new therapies to its Commissioner’s National Priority Voucher program, bringing the total to 15 products that address major public health needs, including obesity, cancer, sickle cell disease, and drug-resistant tuberculosis.
The FDA has approved a new single-injection, once-monthly maintenance regimen for Eli Lilly’s Omvoh (mirikizumab-mrkz), offering adults with moderately to severely active ulcerative colitis a more convenient dosing option that maintains proven efficacy and long-term remission outcomes.

The FDA has updated the label for Merck’s Winrevair (sotatercept-csrk) following results from the Phase III ZENITH trial, confirming the therapy’s ability to reduce the risk of clinical worsening events in adults with pulmonary arterial hypertension.

Lynkuet, a hormone-free therapeutic option for hot flashes associated with menopause, is expected to be available in the United States in November 2025.

Amgen and AstraZeneca’s Tezspire (tezepelumab-ekko) gains FDA approval as an add-on maintenance therapy for patients aged 12 and older with inadequately controlled chronic rhinosinusitis with nasal polyps.
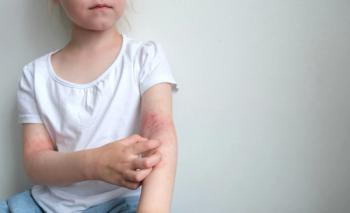
The FDA has expanded Johnson & Johnson’s Tremfya (guselkumab) approval to children six years and older weighing at least 40 kilograms with moderate to severe plaque psoriasis or active psoriatic arthritis, marking the first pediatric approval of an IL-23 inhibitor for these conditions.
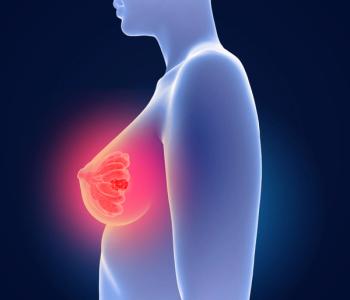
FDA approves Eli Lilly’s Inluriyo (imlunestrant) for adults with ER-positive, HER2-negative, ESR1-mutated advanced or metastatic breast cancer that has progressed after endocrine therapy.
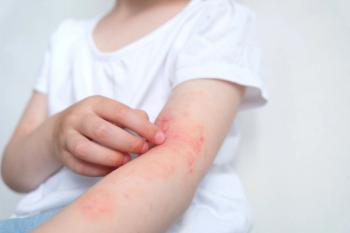
The FDA has expanded the indication of Incyte’s Opzelura (ruxolitinib) cream to include children aged two to 11 years with mild to moderate atopic dermatitis, making it the first steroid-free topical treatment option for this age group.

FDA approves COVID-19 vaccines from Moderna, Pfizer-BioNTech, and Novavax targeting the LP.8.1 sublineage of SARS-CoV-2, with eligibility limited to adults aged 65 years and older and those with underlying medical conditions that place them with a high risk for severe disease.
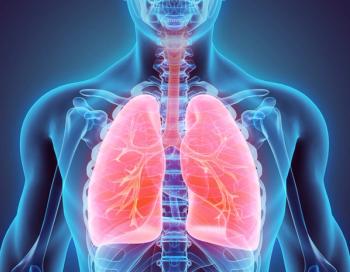
Brinsupri is a first-in-class DPP1 inhibitor that has been found to inhibit activation of neutrophil enzymes that drive chronic airway inflammation in non-cystic fibrosis bronchiectasis.

Regeneron has received a second complete response letter from the FDA for odronextamab and anticipates delays for Eylea HD due to inspection findings at a Novo Nordisk-owned manufacturing site, despite continued strong revenue growth and pipeline momentum.

Following a complete response letter rejecting accelerated approval for RP1 in advanced melanoma, IGNYTE trial investigators are urging the FDA to reevaluate the therapy’s robust survival data, just as agency leadership changes raise new questions.
AbbVie secures FDA approval for Rinvoq as the first oral Janus kinase inhibitor indicated for giant cell arteritis, expanding its immunology portfolio and signaling strategic growth opportunities in underserved autoimmune markets.

The FDA has granted Fast Track Designation to Adicet Bio’s ADI-001, an allogeneic gamma delta CAR T-cell therapy, for refractory systemic lupus erythematosus with extrarenal involvement, marking the second such designation in autoimmune diseases.

Roche’s Susvimo, a refillable eye implant for diabetic macular edema, provides continuous delivery of ranibizumab, showing sustained vision improvements with fewer treatments than standard eye injections.
The FDA has approved an expanded label for Roche’s PATHWAY anti-HER2/neu (4B5) Rabbit Monoclonal Primary Antibody test, which identifies patients with HR–positive, HER2-ultralow metastatic breast cancer who may be eligible for treatment with Enhertu.

Johnson & Johnson has submitted a supplemental Biologics License Application to the FDA for a subcutaneous induction regimen of Tremfya for adults with moderately to severely active ulcerative colitis based on positive Phase III ASTRO trial results.

Jazz Pharmaceuticals' Ziihera (zanidatamab-hrii) provides a chemotherapy-free treatment option for patients with HER2-positive biliary tract cancer, addressing a critical unmet need with promising efficacy and improved quality of life.

Bimzelx is the first approved medication for active psoriatic arthritis, non-radiographic axial spondyloarthritis, and ankylosing spondylitis that selectively inhibits IL-17A and IL-17F, both of which are key cytokines that drive inflammatory processes.

AstraZeneca’s Farxiga has previously been approved by the FDA as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus, in addition to indications for heart failure.
Breyanzi, a CD19-directed CAR T-cell therapy, granted fourth FDA approval to treat a distinct subtype of non-Hodgkin lymphoma.

This is the second accelerated approval the FDA has granted to Bristol Myers Squibb’s chimeric antigen receptor T-cell therapy in the past few months.
Ojemda is the first systemic therapy approved by the FDA for the treatment of relapsed or refractory pediatric low-grade glioma harboring a BRAF fusion or rearrangement, or a BRAF V600 mutation.

Published: December 15th 2023 | Updated: February 22nd 2024

Published: November 27th 2023 | Updated: February 22nd 2024

Published: December 13th 2023 | Updated: February 22nd 2024

Published: December 14th 2023 | Updated: February 22nd 2024

Published: August 1st 2025 | Updated: August 7th 2025

Published: January 15th 2024 | Updated: February 22nd 2024