Compliance as Usual Doesn’t Cut it Anymore
Pharmaceutical Executive
How pharma companies can bolster their quality management programs to meet FDA’s evolving compliance requirements.
The "Quality" Imperative: Facing FDA’s evolving and “continuous improvement” compliance requirements, the need for pharma companies to measure quality, so they can effectively manage it, is paramount
Pharmaceuticals executives and health authorities have always recognized that organizational quality is inextricably linked to compliance. However, more recently, regulators have been shifting from a primary compliance focus to more of a continuous improvement and quality focus. The challenge is to keep the pharmaceutical industry’s investments in quality and compliance in lock-step with regulators’ focus and expectations.
Historically, pharmaceutical manufacturers have been “checking the boxes.” Given the continued success and pace of drug product introduction and availability, they’re generally achieving compliance. At the same time, continuous improvement efforts by health authorities like FDA have continued over the last few decades in the form of revamping and releasing regulations. Likewise, manufacturers have stayed in step to comply with the new regulations. The expected result of health authorities’ and manufacturers’ continuous improvement is less recalls, alerts, and shortages-but that has not been the case.
The imperfect correlation between compliance and drug quality is not a recent discovery. In 2002, FDA created the “21st Century Initiative,” which included an assessment of the correlation between current good manufacturing practice (cGMP) regulations and quality systems. In 2006, the initiative resulted in a guidance for a quality system approach suggesting that “an effective quality system, by lowering the risk of manufacturing problems, may result in shorter and fewer FDA inspections.” Continuing the pursuit of higher quality, FDA launched the “Case for Quality” in 2011, and in 2014, the agency created the Office of Pharmaceutical Quality.
Manufacturers are in agreement with regulators, as they too have emphasized a focus on quality for years now. Initiatives such as “Quality by Design” and “Culture of Quality” are common across the industry. Sparta Systems’ 2018 survey on Pharma Quality Outlook reported that quality teams are taking on expanded roles such as “driving operational efficiency, improving manufacturing performance, and accelerating time to market.”
The life sciences industry recognizes the key elements of an effective pharmaceutical quality system as:
- Process performance and product quality monitoring system
- Corrective action/preventive action (CAPA) system
- Change management system management review
But a challenge that has yet to be met is how to measure quality, and thus how to effectively manage it.
At a 2014 conference at Xavier University, Russell Wesdyk, scientific coordinator for FDA’s Office of Pharmaceutical Science, explained that there are no objective measures for tracking quality improvement. He said, “We don’t even have a definition of what ‘quality’ means. Quality standards just don’t exist.”
Measuring quality can help manufacturers toward their goal of producing higher-quality products, as well as relieve some of the regulatory pressure for compliance. For example, Wesdyck explained that industry metrics

can assist FDA in prioritizing their field investigations.
In 2015 and again in 2016, FDA’s quality metrics program released guidance to gather industry feedback to inform the agency on how best to implement a widespread reporting program. FDA recognizes that by adopting a quality measurement program, companies will improve their overall quality systems.
A 2018 Sparta Systems survey reported that only half of respondents anticipate using quality data to “improve manufacturing performance” during the next 12 to 16 months. One explanation for why the industry is still struggling to leverage quality data is that it is still not well-defined and remains difficult to gather. Off-the-shelf pharmaceutical quality system (PQS) and quality management system (QMS) tools do include some quality metric reporting, but it is not comprehensive. Quality metrics should reflect quality as it relates to product speed to market, effectiveness of processes and functions, organizational culture, and, of course, patient well-being.
Measuring all these elements of an organization’s quality requires gathering data from many sources beyond just the PQS and QMS. Valuable quality data also lives in sources such as:
- Electronic data management systems (EDMS)
- Clinical trial management systems (CTMS)
- Regulatory information management systems (RIM)
- Enterprise resource planning systems (ERP)
- Marketing/sales intelligence systems
- Budget impact models (BIM)
- Manufacturing execution systems (MES)
- Electronic drug accountability system
- Finance/accounting systems
- Interactive voice/web response systems (IVR/IWR)
- Electronic data capture systems (EDC)
- Electronic case report form systems (eCRF)
- Learning management systems (LMS)
- Inform accountability and compliance through on time metrics
- Compliance management system
- Enterprise MS outlook
- Complaint management systems
- Human resource information system (HRIS)
- Correspondence management
- Audit/inspection management
At first look, some of these sources (e.g., EDMS and LMS) do not appear to hold quality data. However, an EDMS stores milestone data that informs the speed and effectiveness of the regulatory processes, and an LMS informs accountability and compliance through on-time training metrics.
Near real-time quality analytics metrics can empower an organization to fully realize a culture of quality. With standardized scores, executive leadership and functional heads can measure daily and control the quality, identifying problematic trends and root causes sooner (see figure).
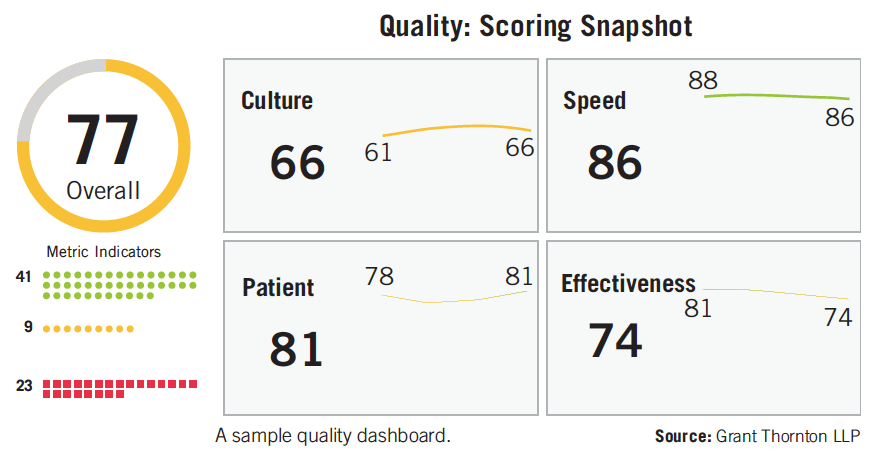
FDA is depending upon the industry to inform its quality metrics program. Comments from manufacturers, along with monitoring and collaborating on quality analytics dashboard development efforts, will form the basis for future metrics reporting requirements and prioritization of FDA inspections. When Wesdyk was asked in 2014 if reporting good quality metrics would cancel an FDA inspection, he replied “It’s too early to tell. But certainly plants ranked high [for quality] would not be on a priority list to inspect.”
In addition to relief from the burden of inspections from the FDA incentive programs, companies with effective quality management programs have improved top-line and bottom-line performance. Hence, those organizations that engage in the development and usage of these analytics will likely have a competitive advantage over their peers. Compliance will become a natural byproduct of a culture of quality, where quality and continuous improvement is business-as-usual. Without effective measurement, continuous improvement will struggle and compliance issues will continue to haunt the industry.
Brad Pedrow is Director, Compliance Risk, Life Sciences, Grant Thornton LLP

MDMA Therapy for Mental Health Conditions: Do the Benefits Outweigh the Risks?
October 25th 2024Despite a recent FDA Complete Response Letter issued to Lykos for midomafetamine capsules for the treatment of post-traumatic stress disorder, experts believe that the future is bright for psychedelic drugs that treat mental health conditions.
Securities Litigation Arising from Alzheimer's Drug Treatments
September 25th 2024The legal challenges surrounding Biogen’s Aduhelm and Cassava Sciences’ simufilam underscore the ongoing difficulties in Alzheimer's drug development, leading to securities litigation over allegedly misleading statements about trial results and commercialization efforts.