Culture Clubs: Independent Ethics Committees
Pharmaceutical Executive
Before clinical trials began to cross international lines, recruiting patients was fairly simple. Recruiters had to research only one country's cultural and regulatory standards. But as trials go multinational and the various players in the global study community begin working together for the first time, sponsors discover new levels of complexity. Divergent regulations, cultures, and languages hamstring efforts to find a single approach to patient-outreach communications.
Before clinical trials began to cross international lines, recruiting patients was fairly simple. Recruiters had to research only one country's cultural and regulatory standards. But as trials go multinational and the various players in the global study community begin working together for the first time, sponsors discover new levels of complexity. Divergent regulations, cultures, and languages hamstring efforts to find a single approach to patient-outreach communications.
What will work in Italy, may not be culturally acceptable in Romania, legal in South Africa, or meet ethical standards in the United Kingdom. Companies recruiting patients need to localize outreach materials for each national population. To meet a country's ethical standards, recruiters have to understand the regulatory culture first, which means they have to learn more than the law. Global standards remain a tiny speck on the horizon, so companies are facing an uphill battle to determine, on a country-by-country basis, the appropriate way to approach potential patients.
Ethics committees and their review systems are central to the developing global regulatory climate. Like their US and Canadian counterparts, called Institutional Review Boards (IRBs), ethics committees are independent groups, comprised of both medical and non-medical personnel. They are charged with ensuring the protection, safety, and well-being of people participating in clinical trials. Committees review and approve the study protocol, investigators, facilities, and methods, and examine all materials to be used in obtaining and documenting informed consent of study participants. This includes patient-recruitment communications.

With different ethics committees often monitoring the same trial in several countries, compliance becomes a worldwide job. Typically, global study managers have little knowledge of regulatory or other recruitment conditions in countries outside their own, where trials take place. Recognizing that global patient-recruitment efforts hinge on accurate expectations about regulatory and ethical standards, BBK initiated an online survey of ethics committee members in more than 60 countries in Europe, Asia, and South America. Using local contacts and key opinion leaders to contact the most influential and experienced people available, BBK compiled a guide to precedents and preferences for patient-recruitment strategies around the world.
The ongoing survey, initiated in 2005, has already uncovered some interesting data about the basis for approving or prohibiting certain communications tactics (e.g., television and print advertising, posters, publicity, brochures, referring physician outreach, etc.) within a country. While regulatory guidelines play a role in this process, cultural preferences and even the individual choices of ethics committee members, are seen to play a larger one. In some cases, a committee member's belief that an outreach strategy like Web advertising is not allowed—or at least not appropriate—rests only on the fact that no one has done it before. Having a rough guide to the views of ethics committees around the world can help shape conversations with country study managers, who, like ethics committee members, sometimes reject vehicles for patient recruitment outright, even though they rarely can point to a law or regulation as justification.

Culture vs. law: respondents who relied on culture (beliefs, convention, and precedent) to determine viability of tactics vs. those who believed a specific law prohibits particular tactics
Surprising Findings
The most startling finding to date from BBK's survey is that ethics committees appear to have almost capricious systems—rather than clearly established guidelines—for reviewing materials. Even within the same country, committees have been inconsistent about what tactics are allowed or disallowed. The justifications committee members give to support their decisions are varied, inconsistent, and contradictory. Consider the following examples.
Advertising
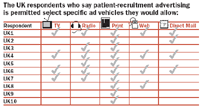
The survey asked respondents to indicate to what extent advertising was permitted as part of a patient-recruitment campaign. Of respondents from the United Kingdom, 94 percent checked "some forms of advertising are permitted," while six percent marked "convention disallows advertising." Of those six percent, all acknowledged that although they believe advertising is not allowed, they could cite no law that prohibits advertising for patient recruitment. But even though most UK respondents clearly believe that advertising is permissible, the picture gets murky when respondents are asked what specific ad vehicles are allowed. While most believe print is acceptable, television, radio, Web, and direct mail advertising all received mixed responses. Based on survey comments, it seems clear that respondents are reacting to each medium based on what media they've actually seen used before, and perhaps on their own personal ideas and preferences.
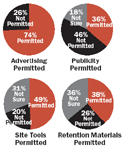
Ethics committee members around the world approve various patient-outreach tactics, but many are unclear about why they do so. As was the case for advertising media, such standard materials as site tools, press kits, and retention materials fall into a gray area, where culture trumps law.
Respondents in other countries sent mixed messages as well. In Belgium, 43 percent of ethics committee members responded that the full range of ad vehicles is permitted. Of the 57 percent of members who said advertising is "not permitted," half cited "no law" prohibiting advertising, while the other half indicated they believe there is a law that prohibits use of ads. Respondents in Spain split evenly on the question of whether advertising is allowed, indicating either that the full range of advertising tactics is allowed, or that none is.
In a sampling of respondents from a range of countries, it becomes clear that advertising for patient-recruitment purposes and the forms it takes is inconsistent. The common denominator seems to be that ethics committee members more often use convention and precedent in their judgment of allowable tactics, rather than knowledge of concrete regulations.
A Question of Semantics and Perception
It may be that the word "advertising" elicits confusing responses from ethics committee members. The term itself, which could bring ideas like "coercion" and "selling" to mind, has different connotations for different people. Some reject it entirely. For others, individual media may provoke strong feelings and preferences. Take direct mail, for example. In Finland, for example, ethics committee members say that sending a postcard to inform a person about a clinical trial for diabetes is too intrusive, since it informs recipients that researchers know they have diabetes. In Slovenia, on the other hand, direct mail is considered a discreet and acceptable approach. Some may think of TV as benign and all encompassing, while others may view it as too coercive—indeed, the most coercive medium. And in countries where radio is historically a public, commercial-free medium, radio advertising of any sort is still new, which may slow the acceptance of radio recruitment ads.
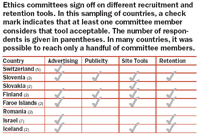
In BBK's survey, some respondents who indicated they believe advertising is permitted stopped short when asked to select specific vehicles—perhaps another indication of general confusion on the subject.
Also confusing the matter: In the EU, advertising for reimbursable medicinal products is banned by law, so ethics committee members may be unwittingly extending these laws to patient-recruitment advertising—unnecessarily forfeiting the benefits these tactics could provide, like enrolling studies faster and speeding up time-to-market for new treatments.
In the European Union Directive (2001/20/EC)—the legislation intended to align the EU with international standards for conducting clinical research and to encourage innovation—Article 8 uses the word "advertising" to refer to patient recruitment. It explicitly acknowledges the role of direct-to-patient outreach and education in furthering clinical research and development.
This fact alone should inform ethics committee members that the EU is broadening the definition of advertising for patient-recruitment purposes to include ethical, non-coercive campaigns focused on awareness and education. However, respondents to BBK's survey, when asked about the impact of the Directive on direct-to-patient outreach, appeared to be noncommittal. Most said that they had "not considered" its implications—an indication that the process of adopting and integrating these standards throughout the clinical research community is a slow one, especially if sponsor companies do not push the issues.
Other Patient-Outreach Communications
Another series of survey questions asked ethics committee members to respond to additional patient-recruitment communications, including publicity (press kits), site-based outreach materials, and retention materials, such as low-cost health-oriented items for patient recognition.
Again, ethics committees disagree about which communications tactics are allowed by law. The vast majority of ethics committee respondents who indicated that a particular tactic was not permitted also said they knew of no law that prohibited the use of that tactic. And many ethics committee members who called tactics permissible appeared to base their responses not on an awareness of specific regulatory guidelines, but on the fact that they had seen them used before. As was the case with advertising, convention, precedent, and personal choice seem to have a greater impact on committee decisions than regulatory stipulations.
Implications
The wildly divergent answers from ethics committee members presents a challenge to multinational patient recruiters, but they open up some intriguing opportunities as well. The good news to take away from BBK's survey is that worldwide regulatory attitudes about tactics and patient outreach in clinical trials may actually be more favorable than most people, including country study managers and ethics committee members, think. The pharmaceutical industry can seize an opportunity to educate the clinical study community—including country study managers and ethics committees—on a broader definition of "advertising," to include building patient awareness about clinical trials.
Because ethics committees rely on cultural assumptions, they are open to cultural change. By firmly but sensitively presenting new patient-recruitment tactics wherever the opportunity emerges, sponsors can speed patient recruitment while keeping it culturally appropriate and within the bounds of ethical standards.
Instead of asking, "Can we use a specific communications tactic?", pharma should ask, "Do we need to use the tactic?" If the answer is "yes," the best bet is to submit the communications materials to the ethics committee, supported by a strong and specific argument. Working closely with the country study manager, global trials managers can choose the most appropriate tactics, without caving in to assumptions and conventions. The only way to expand the boundaries is to push the boundaries, and see what gives way.
As global clinical trials evolve during the next decade, they provide an opportunity for sponsors and sites to continue pushing the boundaries. Researching and respecting cultural and ethical guidelines for each country is vital. But the goal is to challenge those unreliable assumptions and unquestioned precedents that tend to limit patient recruitment. The more recruiters learn about ethics committees, the more opportunity they have to leverage each country's desire to reap the rewards of clinical research, which is the best motive for effecting cultural and regulatory change.
Matthew Kibby is a lead member of the strategic services group at BBK Healthcare, Inc. He can be reached at mkibby@bbkhealthcare.com

FDA Outlines Updated Requirement for Placebo-Controlled Trials in Vaccine Research
May 21st 2025In an article recently published by The New England Journal of Medicine, FDA higher-ups Vinay Prasad, MD, MPH; and Martin A. Makary, MD, MPH, wrote that any new COVID-19 vaccine must now be evaluated in placebo-controlled studies.
Addressing Disparities in Psoriasis Trials: Takeda's Strategies for Inclusivity in Clinical Research
April 14th 2025LaShell Robinson, Head of Global Feasibility and Trial Equity at Takeda, speaks about the company's strategies to engage patients in underrepresented populations in its phase III psoriasis trials.
Undertreated but High-Risk: The Hidden Health Crisis Facing 50+ Americans
May 15th 2025Leandro Boer, MD, PhD, VP, US general medicines, Amgen, explains why many high-risk Americans aged 50+ remain undertreated for cardiovascular disease, citing insurance gaps, risk misperceptions, and complex guidelines.
Key Findings of the NIAGARA and HIMALAYA Trials
November 8th 2024In this episode of the Pharmaceutical Executive podcast, Shubh Goel, head of immuno-oncology, gastrointestinal tumors, US oncology business unit, AstraZeneca, discusses the findings of the NIAGARA trial in bladder cancer and the significance of the five-year overall survival data from the HIMALAYA trial, particularly the long-term efficacy of the STRIDE regimen for unresectable liver cancer.
Novo Nordisk’s Sogroya Demonstrates Efficacy in Children with Growth Disorders
May 14th 2025Results from the Phase III REAL8 basket study found that once-weekly Sogroya was effective in children with growth disorders, including those born small for gestational age, with Noonan syndrome, or idiopathic short stature.