
Could the new Vermont Drug Transparency law “lead the US Pharmaceutical industry to an awful end”? Tom Norton reports.
Tom Norton is Principal at NHD Smart Communications of Illinois, Inc.

Could the new Vermont Drug Transparency law “lead the US Pharmaceutical industry to an awful end”? Tom Norton reports.

California is in the process of potentially establishing a new, disruptive healthcare policy that is proving to be very concerning to the American Rx industry, writes Tom Norton.

Even though pharma's “dark days” are fading away, the chill of the last few months will not soon be forgotten, writes Tom Norton.

As US pharma continues to ponder its “winter of discontent”, the question that leaders of industry have to be asking themselves is, “How did things get this bad?” Tom Norton reports.

As that nation heads towards its first presidential primaries of the 2016 election, pharmaceutical executives should take a few minutes to consider where the leading candidates stand on U.S. Rx policy. Tom Norton takes a look.

What are the chances that Rx price controls will actually take hold in the U.S. in the near future? Tom Norton believes the answer to that question can summed up in one word: California.
Hillary Clinton has been a part of the healthcare debate since the early 90's. Tom Norton analyzes her career of positions on drug price control and what it could mean for her candidacy.

The issue of drug price controls in public prescription programs has long been a point of contention. But today, writes Tom Norton, as several historical and contemporary factors continue to line up, we may be on a pathway to changing that.

For the second time in three years, the Supreme Court of the United States (SCOTUS) has weighed in on key aspects of the 2010 Obamacare law. Tom Norton reports.

For the 17 states that set up their own state exchanges under Obamacare, finding ways to manage a couple aspects of the new law are turning 2015 into a “Year of Harsh Realities.” Tom Norton explains why.

Watching the latest volleys fly in the ongoing specialty drug pricing wars, Tom Norton wonders: Is it possible that all involved couldn’t just step back and take a breath?

At first glance Obamacare developments for the American Rx industry in 2015 appear fairly benign. However, digging deeper suggests that each, in its own way, could cause various actions with potential for substantial uncertainty for the U.S. pharmaceutical industry next year. If your response to this profound observation was, “Really, what’s new?” - read on.

While flipping through my emails recently, I froze before an image that appeared on my screen. An old Rx colleague had sent me a graph, mapping out the 2014 U.S. sales achieved by Gilead’s Hep C drug, Sovaldi. It was stunning.

It’s fall and the 2015 Obamacare Exchange Rx insurance offerings will be presented to patients on November 15th. But at this point, what insights do American Rx brand managers and marketers actually have on the results of the Obamacare Rx experience in 2014?

In the wake of last week’s declaration by new HHS Secretary, Sylvia Burwell, that “Obamacare is working”, it’s probably time for American Rx product managers and marketers to step back and consider where we have travelled since January 1st of this year.

As the “Saga of Sovaldi” continues to unfold in the U.S., and Congress, insurers, providers, and the U.S. Rx manufacturer, Gilead, hurl charges back and forth at each other, it’s pretty clear the situation will only continue to deteriorate.
As the "Sovaldi Saga" unfolds in the U.S., it's clear the situation will only deteriorate. But north of the border, it's striking how Canadian healthcare entities are managing a similar Sovaldi situation, writes Tom Norton.

PCMA represents several American managed care entities and has been an active participant in the national debate over the issue of high priced biotech Rx drugs.
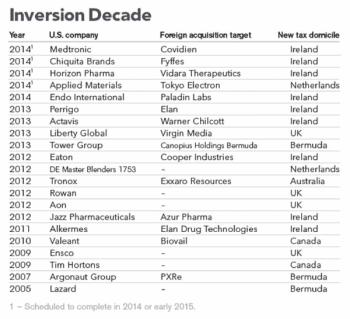
As I read the latest reports of yet another big US Rx firm, this time AbbVie, trying to pull off a “tax inversion” takeover of the Irish-based Shire, I couldn’t help but wonder, what the heck is going on here?

Tom Norton outlines the Obamacare-related questions that are likely to be of concern to pharma companies in the U.S. this year.

So, we’ve experienced two months of Obamacare. How is the U.S. pharmaceutical industry doing with this new program? I spent two weeks getting the opinions of several Rx representatives on their experience with Obamacare, as well as their thoughts on how it is impacting their marketing, sales, and R&D planning for 2014 and beyond.

In the early 1990’s, during the debate over the Clinton healthcare plan, I recall sitting in an interesting meeting at my pharma company headquarters.

A significant, though as yet not fully understood player in the launch of Obamacare is the pharmacy benefit manager (PBM) industry.

For more than three years, the US pharmaceutical industry has known that today would arrive. So this is it. The first day that eligible U.S. citizens can sign up for Obamacare - and enjoy the prospect of prescription drug insurance, effective January 1st, 2014.

We are now less than three weeks away from the initial sign up period for Obamacare. After October 1st, the reality of the program and all that it portends for the future of the US pharmaceutical industry will begin to make itself apparent

As many of you may recall, earlier this year I commented on a section of the new Obamacare law that directed Members of Congress and their staffs to participate in the new healthcare program, effective January 1, 2014.

If you are an Rx regional sales director or a product marketing manager, I have a somewhat provocative question for you: How exactly are you planning your 2014 Medicaid strategy?

This past week or so, with Obamacare’s start up less than a year away, we have been treated to several entertaining vignettes as the nation’s governors begin to wrestle with the realities of the new healthcare law.

With the general election behind us, the anticipated avalanche of ObamaCare regulations has begun. Last week, it was reported that more than 13,000 pages of rules and regulations have been issued by HHS since Nov. 6th

If you’re like me, you probably bounded out of bed on Thursday morning, took in all the news you could find about the Supreme Court and healthcare reform…and then you waited.
Published: July 30th 2014 | Updated: November 17th 2020

Published: December 8th 2014 | Updated: November 15th 2020

Published: November 18th 2014 | Updated: November 15th 2020

Published: October 15th 2014 | Updated: November 15th 2020

Published: October 1st 2014 | Updated: November 15th 2020

Published: September 5th 2014 | Updated: November 15th 2020