
Pharmaceutical Executive
The top 50 pharmaceutical companies, ranked by prescription pharmaceutical drug sales, plus rankings of top products, R&D spend, and more.

Pharmaceutical Executive
The top 50 pharmaceutical companies, ranked by prescription pharmaceutical drug sales, plus rankings of top products, R&D spend, and more.

Pharmaceutical Executive
As the industry focuses its attention on the upcoming renewal of the Prescription Drug User Fee Act (PDUFA), there is a tendency to overlook two other significant pharmaceutical programs coming up for renewal and a related piece of legislation that has been introduced:

Pharmaceutical Executive
Perhaps the biggest mistake a first-time leader can make is waiting until the job actually begins before going to work.
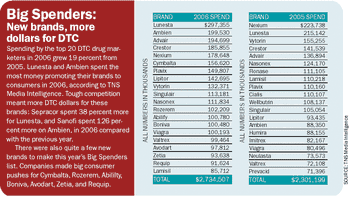
Pharmaceutical Executive
Direct-to-consumer advertising officially becomes a "tweenager" this August-and, oh my, how it has grown. DTC was officially born in 1997 when FDA gave the green light to companies to advertise their drugs to consumers. In the first year, pharmaceutical marketers bounded onto the scene and spent more than $1 billion. Years passed. Debates ensued. Patients learned more about drugs. And, yes, spending grew. The latest available figures for 2006 show that the industry spent $4.8 billion on DTC advertising, a 13 percent increase over 2005 and the second year of double-digit growth.

Pharmaceutical Executive
When it comes to corporate reputations, it's clear the pharmaceutical industry doesn't quite get it. (Just pick up any newspaper.) But here's the latest newsflash when it comes to managing a bad rap: You get what you pay for.

Pharmaceutical Executive
When it comes to direct-mail marketing, tiny envelopes and wordy letters just don't cut it. But for a fraction of the cost of a sales call, you can create an eye-catching mailer that will land on physicians' desks rather than in the circular file.

Pharmaceutical Executive
In march, us rep. rahm "Mr. Television" Emanuel (D-IL) reintroduced legislation aimed at what he calls "driving down the price of prescription drugs." But the only thing such legislation would accomplish would be the "driving down" of pharmaceutical innovation.

Pharmaceutical Executive
Pharma industry alliances are increasingly critical to success. They are gaining on R&D in terms of importance, especially since many companies are falling short in their internal pipelines and need to look outside for promising compounds. Today, nearly two-thirds of the top 20 pharma and biotech companies have established alliance-management functions. These usually cover deals in the discovery, development, and marketing space.

Pharmaceutical Executive
The push is on to establish an approval pathway for generic versions of biotech therapies. The Hatch-Waxman Act of 1984 established a process whereby generic-drug manufacturers could obtain approval for a product based on the innovator company's data. But Hatch-Waxman doesn't apply to biologics regulated by the Public Health Service Act, and generics makers-as well as some Big Pharma companies and small biotech firms-want Congress to give the Food and Drug Administration authority to set up a similar process for these products as well.

Pharmaceutical Executive
It's not always obvious just who is reponsible for the various steps of pharmacovigilance.

Pharmaceutical Executive
Over the past 20 years, drug companies went from having carte blanche to set drug prices to operating in an ever more tightly controlled environment. Instead of doctors calling the shots, government and private payers are becoming increasingly vocal about which drugs they will and will not cover. Frustrated patients, in turn, are getting anxious about their out-of-pocket costs and access to the medicines they need.

Pharmaceutical Executive
Today's highest science, most fierce competition-and make no mistake, biggest gains-are coming from the oncology arena. In fact, there are more than 380 cancer compounds in development, according to a recent report by IMS Health, with almost 100 of them in Phase III trials.