Building Blockbusters
Companies face a serious pipeline gap, partly because they focus too narrowly on scientific breakthroughs. Stakeholders also value convenient compounds with reduced side effects and fewer doses.
Innovation is the lifeblood of the biopharmaceutical industry. New medicines pharma brings to market save lives, reduce suffering, and, not incidentally, generate new revenue streams. Today's increased emphasis on innovation—discovering breakthrough medicines that provide significant therapeutic advantages—is driven in part by the growing resistance of both public and private payers to pay premium prices for medicines that are not demonstrably superior to those already available.
In the United States, pharmacy benefits managers and managed care organizations have adopted tiered formularies and other cost-containment measures that relegate new drugs to a lower reimbursement status unless a significant advantage over existing medicines can be demonstrated. In Europe, a new drug may only receive reimbursement at the level of the lowest-cost alternative—perhaps a generic—if superiority cannot be demonstrated.
The biopharmaceutical industry is responding by focusing on "better" medicines—drugs with novel mechanisms of action that target new diseases or treat familiar ones in new ways. While such drugs may have real therapeutic and financial advantages, they present substantially higher risks in terms of technical success. That is, they are much more likely to fail in development. As a result of this higher level of technical risk in their pipelines, pharmaceutical companies are launching fewer new molecular entities (NMEs) than at any time in recent memory.
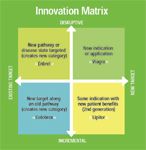
Innovation Matrix
Taking on additional scientific risk is not the only path to innovation. In many cases, companies have defined innovation too narrowly by focusing on pure scientific novelty. This, in turn, has reduced R&D productivity and is likely to erode shareholder value. But evidence shows that focusing on first-in-class drugs may not be the best strategy for all companies.
First, it is important to recognize that not all innovation is purely scientific. Innovation is in the eye of the beholder, and in the medicine, there are at least four beholders: patients, physicians, regulators, and payers. These stakeholders do not always focus on first-in-class medicines. They value innovations in safety, reduced side effects, convenience, and dosing schedule. Such improvements can also speed drug approval, promote physician prescribing, and enhance patient compliance.
Blockbusters arise from various commercially important innovations. The "Innovation Matrix" framework has two axes. The vertical axis, disruptive versus incremental, shows the degree to which a new medicine reshapes the therapeutic landscape, whether or not it is a scientific breakthrough. Disruptive drugs trear maladies witout available, adequate therapies. In some cases, these drugs create new markets or product classes.
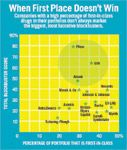
When First Place Does not Win
The other axis shows whether the medicine has a , new target versus existing target, shows the extent to which a new drug is acting on a known target, for which medicines are already available, or has a novel mechanism of action.
The examples given, and there are many others, show that important medicines—those that add value to the company—arise from all four quadrants.
At one extreme, there is Enbrel, a drug that combined a novel mechanism of action with a reshaping of the therapeutic landscape. It has become a blockbuster, with 2004 sales of nearly $2 billion, according to the manufacturer's Web site. For this type of molecule, there is a high degree of technical risk, because it relies on a novel mechanism of action. The commercial risk tends to be lower, because there is no demonstrable therapeutic alternative.
Lipitor lies at the other end of the spectrum. The fifth statin entered a market that was well defined, with a biochemical target similar to its competitors: HMG-CoA reductase. Yet it has become the best-selling drug on the planet. For a molecule such as this, the technical risk was significantly lower. But it carried a higher level of commercial risk as a late entry into a market whose existing products already performed well.
Drugs like Lipitor show that a single-minded focus on new mechanisms of action or first-in-class drugs is not the only—or even the best—way of generating commercial value. Firms with high proportions of first-in-class drugs in their in-line portfolios are not the best at creating blockbusters (see "When First Place Doesn't Win").
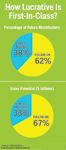
How Lucrative Is First-In-Class?
First-in-class will not guarantee blockbuster status in the future, either. Consider the 21 pipeline compounds deemed (in 2003) to have blockbuster potential (see "How Lucrative Is First-In-Class?"). Two-thirds of the compounds, and most of the forecasted revenues, come from so-called non-innovative drugs, or follow-on compounds. Of the 13 follow-on compounds reviewed in 2003, 11 (85 percent) have been launched or are still in clinical development. Of the eight first-in-class compounds, however, only three (38 percent), remain in clinical development or have been launched.
What are the implications for the biopharmaceutical industry? First, pharmaceutical companies need to take a broader view of innovation. Focusing on scientifically risky, but potentially important new medicines must continue. But it cannot be the only road companies follow. Innovation needs to be assessed from the points of view of stakeholders—patients, physicians, regulators, and payers—whose needs can often be met without scientific novelty.
Next, companies need to develop and maintain a robust mix of first-in-class, best-in-class, follow-on, and line extensions in R&D portfolios that are calibrated to their unique situations. Key elements to take into account include:
- The profile of the compounds currently in the portfolio
- A company's unique strengths and competencies: Does it perceive itself to be a marketing powerhouse able to sell drugs that are only moderately differentiated? Or must it rely on novelty to make up for less strength in the sales arena?
- The risk tolerance of the company's management and board. Some companies value predictability above all else, others are willing to gamble on bigger successes.
All of these factors need to be addressed proactively. Otherwise, a company's portfolio-management process will drive its R&D and overall strategy, rather than the other way around.
Pharmaceutical companies face a substantial pipeline gap, in part because they focus too narrowly on innovation as scientific breakthrough. By taking a broader, more comprehensive view of innovation, and balancing their portfolio risk accordingly, companies can raise their success rate and thereby increase the number of drugs they bring to market. Beyond improving commercial returns, companies will lower their overall risk profiles, become more attractive to investors, and enhance shareholder value. This is not to say that companies should minimize the value of scientific innovations. But they should take steps to manage the overall risk balance of their portfolios by deciding in advance how many resources they will allocate to each type of innovation.
Michael G. Parkermichael_parker@monitor.com is global account manager and David Amardavid_amar@monitor.com is partner at The Monitor Group.
The authors gratefully acknowledge the contributions of Daniel Teper and Adriana Samper to the concepts presented in this article.

The Misinformation Maze: Navigating Public Health in the Digital Age
March 11th 2025Jennifer Butler, chief commercial officer of Pleio, discusses misinformation's threat to public health, where patients are turning for trustworthy health information, the industry's pivot to peer-to-patient strategies to educate patients, and more.
Navigating Distrust: Pharma in the Age of Social Media
February 18th 2025Ian Baer, Founder and CEO of Sooth, discusses how the growing distrust in social media will impact industry marketing strategies and the relationships between pharmaceutical companies and the patients they aim to serve. He also explains dark social, how to combat misinformation, closing the trust gap, and more.