
Pharmaceutical Executive
Eyes in the Back of Your Head

Pharmaceutical Executive
Eyes in the Back of Your Head

Pharmaceutical Executive
Pharma trial sponsors and their CROs should enlist the support of high-level executives to make sure designated decision makers are fully confident in their ability to act.

Pharmaceutical Executive
State clinical trials requirements are in place to protect people from being exploited, or unsafely exposed to compounds. Forty years later, it's easy to say, "How did this happen?"

Pharmaceutical Executive
Pharma companies are committed to using electronic data capture in clinical trials. Technology adoption will continue to grow, as FDA and consumers want faster safety data.

Pharmaceutical Executive
All too often, abuse liability and dependence potential are afterthoughts in the drug development process.
Pharmaceutical Executive
If a physician wants to use protected healthcare information (PHI) for research purposes, particularly if the PHI is going to be published as part of research results, an authorization or waiver will be required.
Pharmaceutical Executive
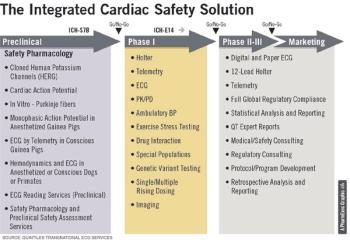
A prolonged QT interval creates an electro-physiological environment that is favorable for the development of cardiac arrhythmias.

Pharmaceutical Executive
CMS envisions studies to show which drugs keep patients out of hospitals or how certain treatments can reduce side effects. Such analysis would support decisions on best practices in using medications.

Pharmaceutical Executive
R&D leaders face the unenviable choice of either dividing their time between science and operations, or focusing on one to the detriment of the other.

Pharmaceutical Executive
Even though data can single out physicians with high marketing upsides, most pharma companies are doing without such high-value data.

Pharmaceutical Executive
Pharma companies want to promote their own, rather than hiring senior people away from competitors or other industries. But given the narrow backgrounds of the coming generation, fewer and fewer managers have the coordination and foresight needed to succeed in a general management job.

Pharmaceutical Executive
Part of the value of a drug comes from the supply chain that protects its integrity. There's a similar supply chain that preserves the value of drug information. And it needs help.

Pharmaceutical Executive
When Jim Dougherty joined Mcgraw-Hill almost 30 years ago, medical journal publishing was just plain different than it is today. The days of the "three-martini lunch" were slowly coming to an end. Yet many companies still determined their ad schedules based on relationships. There was also less competition: without DTC or the Internet, journals garnered larger percentages of pharma's marketing mix. Today, Dougherty is group vice president of McGraw-Hill Healthcare Information and president of the Association of Medical Publications (AMP), an organization of publishing firms in the medical field. Like many of his peers, Dougherty has witnessed-and continues to witness-the transformation of the field. The future is bright, he says, but most certainly uncertain.

Pharmaceutical Executive
Pharma execs and industry analysts say pharma's reputation has improved during the past year. The general public sees things differently. Research says a few select companies are to blame.