Dear eDiary
Pharmaceutical Executive
Electronic patient-reported outcomes tools let trial sponsors enforce recording deadlines and compliance. They help keep subjects honest.
Patient-reported outcomes (PRO) have long been essential sources of data in biopharmaceutical companies' clinical research. But, data gathered from study subjects have never been as reliable as one might hope. Twenty years of research has shown that patients in clinical trials invent data, respond in incorrect formats, or complete multiple entries at once—often long after the episode has occurred.

One such practice is commonly referred to as "The Parking Lot Syndrome," in which subjects sit in their cars prior to site visits and complete two weeks worth of diaries, trying to recall how they felt days ago. When subjects fail to record data close to the time of the event they are describing—whether it's noting how they slept, recording what they ate, or writing down where it hurt and how they reacted to medication—they often discover that such details are as forgettable as last Tuesday's lunch. Faced with an empty paper diary and a deadline, subjects struggle to remember what they did a few days ago. In some cases, they leave the diary blank or only record what they remember. In the worst case, they make up entries.
Electronic patient diaries help make self-reported patient data much more reliable for companies seeking FDA approval of new therapies or medical devices that include patient-reported outcomes.
Advantage Over Paper
Both FDA and the European Agency for the Evaluation of Medicial Products (EMEA) require sponsors collecting patient self-reported data to employ measures to make sure data are timely, accurate, and attributable. This has been especially difficult to do using paper methods. Even so, a review of NDAs by Laurie Burke, director of study endpoints and label development at the Center for Drug Evaluation and Research (CDER), indicated that 30 percent of all clinical trials relied on patient-reported data for primary or secondary endpoints from 1997 to 2002. Market research of biopharmaceutical pipelines indicates that number will grow over the next five years. CenterWatch, a Web site that lists ongoing studies, puts the current figure even higher: three of four trials include some type of patient reporting component. Clearly, it's critical to maximize the quality and integrity of such data.
Not only do electronic patient-reported outcome (ePRO) solutions enable trial sponsors to enforce recording deadlines and encourage prompt compliance, but they also help keep subjects honest. When patients are issued electronic diaries—typically a personal digital assistant (PDA) with specialized software designed exclusively for the trial—the sponsor dictates the time frame during which patients can record data. For example, if the sponsor decides that the morning diary will be available only between the hours of 6 and 9 a.m. to record sleep data, subjects have only that three-hour window to record their entry. If they forget, there is no entry for that morning.
Of course, the PDA can be programmed to help patients remember to record their data. It might buzz to inform subjects that a recording period has begun, or beep to remind them that they have 30 minutes left to make an entry. The sponsor can choose these functions and program them into the study design. At one time of day, only the window for recording sleep data will open. Hours later, that window is closed, but patients can record medication reactions, diet, or physical activity. At the end of the day, data collected on the PDA is transmitted by the subject, through wireless or analog telecommunications, to a central server hosted by the ePRO solution provider. Clinical sites, monitors and sponsors can then review the patient-reported data in real time online. This enables faster decisions and proactive trial management based on up-to-date knowledge of patient diary compliance, key patient data reports, and enrollment. Sites, monitors and sponsors have to wait days or even weeks for double-data entry, reconciliation, and source verification processes associated with paper diaries before they can access the data. If a few days go by without an entry, the site coordinator can call the subject to make sure he or she is using the eDiary properly. With paper diaries, a trial can be nearly over before compliance problems surface.
Worth the Expense?
Despite their apparent advantages, electronic diaries have yet to achieve significant market penetration. Estimates show that less than 12 percent of the studies relying on patient-reported outcomes use ePRO technology. Sponsors who are reluctant to try ePRO sometimes balk at the initial investment or express doubts about the return on investment, according to a 2004 CDSIC/CenterWatch survey. In fact, there is reliable statistical evidence that ePRO can give an immediate return on investment by enabling sponsors to reduce sample size for certain indications.
An insomnia study by Merck Research Laboratories randomized subjects into a paper arm and an eDiary arm, the latter of which used PHT's (proprietary) LogPad System. So far, this is the first and only randomized trial comparing paper and electronic PRO data collection methods in terms of their relative capacity to measure a drug's efficacy. The results demonstrated efficacy for both arms, but the standard deviation for the study's primary endpoint (change in minutes of total sleep time) was 35 percent smaller for LogPad data.
The Merck researchers calculated that such a reduced data variance could enable them to decrease the sample size of future similar trials by 56 percent while preserving the same statistical power. This represents significant potential savings in both time and expense, from patient recruitment to study closeout.
Both paper and ePRO patient groups in the trial had compliance rates over 90 percent, with paper at 96 percent and ePRO at 92 percent. However, many patients did not fill out their paper diaries on time and reported they had to play "catch-up." In addition, the paper data included illogical, illegible, and incomplete entries. Electronic diaries reduced these inappropriate responses, providing sponsors with more valuable data. Real-time access to study and subject data also simplified data management, saving the site 58 hours of data entry.
Geriatrics More Compliant Than Younger Subjects
On-Site Acceptance
Although the potential benefits to sponsors are compelling, some companies worry that patients and investigators may be uncomfortable with the technology underlying ePRO solutions.
"Training subjects is a key element in any ePRO solution," says Darrin Bomba, clinical trials manager at Dynavax. "The first step to improving subject compliance is to make sure site coordinators are comfortable with the technology."
Bomba asks the ePRO provider to train site coordinators directly at investigator meetings. "We budget two to three hours for that training," he says. "Investigators need to be able to help subjects use the eDiaries throughout the study."
An ePRO system involves more than just electronic diaries. User-friendly online tools help sites manage enrollment and diary compliance on a daily basis, and better manage sites around the world.
"It is critical that sites understand not only how, but also why they should use this technology," says Kevin Bayer, a former clinical development coordinator at Orphan Medical. "From a sponsor's point of view, ePRO can improve data quality. But, it can also make life easier for sites. We set up reports so we could see at a glance which sites and subjects exhibited low compliance. This allowed sites to take direct action, such as calling a non-compliant subject before the issue began to affect the outcome of the trial."
Color-coded reports alert sponsors and site managers when a subject's performance drops below certain thresholds. For example, a subject's compliance figure will be shaded red if it drops below 85 percent. The system also can be programmed to automatically alert site personnel to troubles big and small, from changes in a subject's physiologic status to low batteries in a reporting device. Such reports can also be customized. Compiling screening data helps sites make eligibility decisions based on specific criteria, such as the percentage of diaries completed during the enrollment period.
"Another way to ensure that these tools get used is to require it," suggested Bomba. "Language in the clinical protocol can specify that site coordinators review patient compliance daily using the ePRO system." However achieved, whether by training or mandate, the full advantages of ePRO can only be realized if sites leverage the tools at their disposal.
eLearning for ePRO
The ePRO training process typically includes personal interaction and hands-on training for sites at the investigator meeting. Increasingly, training includes a combination of live and online options, including initial Web-based training on basics, prior to the live session, detailed trial-specific training at the investigator meeting, and supplemental training close to the "first patient in" date at a given site. As more sponsors move to online investigator meetings, the virtual training options for making sites comfortable with ePRO solutions will continue to expand.
"Given the scope, expense and logistical requirements of investigator meetings for multi-center studies, conducting training online is a natural solution," says Louis Monti, executive vice president at Pherin Pharmaceuticals. "This is an interactive method to get sites excited about leveraging the tools provided in an ePRO solution."
Online training methods are highly effective and cost-efficient. On-demand access to training modules and product demos are being increasingly leveraged by sites to facilitate subject education throughout the trial.
Maximizing Compliance
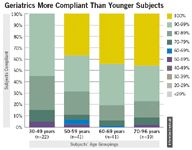
Site coordinators can only do so much. They can't force subjects to comply with the protocol. Many subjects come into clinical trials unfamiliar with handheld technologies, and may at first be anxious about using these sophisticated devices. The two patient populations that tend to worry about this most are pediatrics and geriatrics, despite that geriatric patients up to the age of 90 have exhibited higher compliance in ePRO trials than other populations. (See "Geriatrics More Compliant Than Younger Subjects".)
The best method of dealing with this is with simple designs. "It is important to incorporate simple eDiary designs into your trial protocol," says Dynavax's Bomba. The best interface relies on a single, continually repeated method of entering information. Devices can be customized for geriatric subjects, using larger fonts, touch-screen buttons, and high-resolution displays. Some have even used removable magnifying glasses. For pediatric trials, a caregiver option can be implemented. This allows an authorized caregiver to answer on a child's behalf.
Some ePRO users suggest implementing site-to-subject messages on the PDA to inform patients about how they are doing at certain milestones throughout the trial. Especially during long studies, this helps keep patients' motivation high. These messages appear on the subject's eDiary, and can be archived in the audit trail.
Early Phase Studies
ePRO solutions are used most often to collect data for primary or secondary endpoints in Phase II and III clinical trials. However, Rinat Neuroscience and Greer Laboratories are two innovative companies leveraging an ePRO solution earlier in the research process—as early as Phase I.
"With smaller biotechnology companies, the time to proof of concept is critical," says Patricia Walicke, MD, PhD, vice president of clinical development at Rinat Neuroscience. "We have a strong desire to demonstrate activity in Phase I, and yet we are also resource constrained. Real-time data access and improved ePRO data quality enable us to perform more frequent interim analyses with a smaller group of patients, which keeps costs low and timelines short."
In Greer's case, ePRO is being used to determine the safety and dosing schedule of allergy treatments that are common in Europe, but new to the United States.
"We feel the use of electronic patient diaries in our sublingual oral immunotherapy safety and dosing trials will assist us in collecting and analyzing data in a timely manner," says Brad Whitlow, clinical project manager at Greer.
As clinical budgets shrink and the pressure to push products through the research pipeline increases, drug companies need to save steps. By reducing the errors and inefficiencies that are so common with paper diaries, ePRO solutions enable sponsors to collect compelling and statistically significant data from patients in Phase I through IV clinical trials around the world. This results in better data, more manageable trials, quicker progress to the next phase of research, and ultimately, a shorter road to FDA approval.
Phil Lee is president of PHT Corporation. He can be reached at plee@phtcorp.com.
