
Assessing the implications for the pharma industry of the America Rescue Plan Act of 2021, which provides nearly two trillion dollars in funding for various government programs, including many related to public health.

As the pressure on teams reviewing promotional content has increases, both in terms of volumes of content and complexity, Sameer Lal discusses the need for a near-term strategies using artificial intelligence (AI) and machine learning (ML) to decongest bottlenecks of the manual review process.

Assessing the implications for the pharma industry of the America Rescue Plan Act of 2021, which provides nearly two trillion dollars in funding for various government programs, including many related to public health.

Mitigating potential risk for stakeholders in light of latest Sunshine Act developments.

Treat patents as strategic business assets and build an IP portfolio.
Disputes over mRNA-related patents are poised to persist.

Fighting the pandemic with joint projects and IP sharing.

Exploring practical points to further understand and strengthen quality culture.

Cybersecurity vulnerabilities embedded in AI and machine learning pose significant litigation risks as life sciences companies move to digital technologies.

Pharma manufacturers may need to upgrade their coupon programs to accommodate for the emergence of copay accumulators and related benefit mechanisms.

How the unfolding of notable infringement claim could alter commercial approaches for players in wider CBD treatment field.

How PREP Act protections will apply to potential COVID-19 vaccine-related claims.

Whether your company already has a data-driven compliance program in place or is contemplating building one, Michael O'Connor and Isha Arora outline four things the DOJ is now focused on that your compliance team needs to know.

How pharma leaders can convert data collected for compliance purposes from a cost center into a critical success factor.

Amid the limitations of traditional compliance programs that emphasize control measures, here are steps biopharma companies can implement in their own cultures to ensure they’re behaving and performing with integrity.

Case study illustrates how compliance teams, in today’s climate, can foster innovation across an organization to help achieve corporate goals.

Xavier Duburcq describes how pharma leaders can convert compliance from a cost center to a critical success factor.
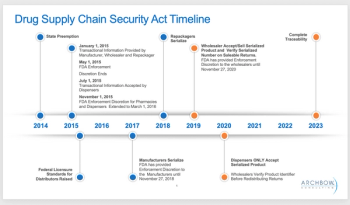
It is vital to begin asking questions about your company’s DSCSA readiness and compliance, as well as that of your strategic partners, writes Rob Besse.

Is Congress harming pharmaceutical research by limiting enforcement of improvement patents?


Op-Ed: The best way to capitalize on the potential revenue influx from enterprise-level drug adherence programs is to create room at the executive table for a Chief Adherence Officer.

James Clark addresses one of the key questions data protection and compliance officers are asking following the implementation of the General Data Protection Regulation -"What role am I playing under the GDPR?"

There’s a need, now more than ever, to balance complex regulations, rising consumer demand and shifting market realities, writes Graham Francis.

Amid a still-difficult environment for enforcing cannabis-related patents, this article explores some of the types of patent protection available for cannabis-based therapies and inventions.

The role of insurance and risk management in protecting middle-market distributors from the growing opioid multi-district litigation (MDL).

Pharm Exec convenes an expert panel at CBI’s Pharmaceutical Compliance Congress to discuss new ways to navigate the many complexities when it comes to the crucial task of assessing business and risk in the life sciences.

Josh Reisberg outlines the foundation of a broad, overall defense strategy for generic companies embroiled in Hatch-Waxman patent infringement litigations.