4th Annual Press Audit: Coverage Uncovered!
Pharmaceutical Executive
Pharma gets less ink as news reporters shift their sights to other topics? That's a good thing--Isn't it?
Ask anyone in pharma if the media's scrutiny of the industry is lessening, and he would most likely answer with a vehement "no." But new research shows that it is—ever so slightly—even as major issues such as drug safety dominate the headlines.

Clipping Service
You might ask yourself, How is that possible? Here's how: First off, there's less media coverage. Compared with 2006, the number of articles written about the pharmaceutical industry in 2007 has been halved. At the same time, many publications have softened their stance, as witnessed through a near doubling of the percentage of articles that positively report on the industry and its positions.
But let's not get ahead of ourselves too quickly: Bad news about the industry continues to command much of the news flow, and keeping an eye on just how the media report on pharma—and how that coverage compares with previous years—remains essential knowledge for executives.
To help the industry keep up, the Arrupe Center for Business Ethics at Saint Joseph's University conducts an Annual Press Audit to identify the issues that attract media attention. This audit, now in its fourth year, uncovered some important findings:
- Media coverage of the industry has dropped 46 percent, with fewer articles in every major newspaper.
- Coverage was still more negative than positive, but less so than in the past.
- Drug safety continues to be the hot-button issue and was included in half of all media coverage.
- Media focus on FDA and regulatory issues continues to intensify, with the number of articles jumping from obscurity in 2005 to number two on the list of most-frequently-covered issues in 2007.
Dissecting the News: Here's How
This press audit analyzed content from the top-five US newspapers as defined by circulation: USA Today, Wall Street Journal,New York Times, Los Angeles Times, and the Washington Post. The audit shed light on the following questions:
- What ethical and legal controversies face the pharmaceutical industry—and what kinds of coverage do they attract?
- Does the media support or oppose positions taken by pharma?
- How often is the industry's perspective included?
- Which pharmaceutical companies and brands are identified and discussed?
To be included in the audit and our EthicsTrak database, an article had to be published between October 1, 2006, and September 30, 2007. It also had to (a) focus on an ethical or legal issue facing pharma and (b) appear either as a front-page story or on the editorial page—an indication of major news and public sentiment. We focused on daily newspapers rather than the broadcast media or weekly magazines for a number of reasons. Dailies can cover a broader range of issues and in more depth than the sound bites reported on radio and TV. Business and news magazines are also constrained by their weekly and monthly format. Further, newspapers provide editorial coverage that takes a specific and unambiguous position—pro or con—toward industry issues.
For each article, we examined four elements:
Issues We categorized the issues (as defined by PhRMA) that were discussed in each article. Many articles covered two or more issues and were included in relevant sections.
Headline We categorized the headlines as positive, negative, or neutral toward pharma. For example, "Is Your Doctor Tied to Drugmakers?" (New York Times, June 2, 2007) was classified as a negative headline, while "Yes, New Drugs Saves Lives," (Washington Post, July 11, 2007) was labeled positive.
Tone We also analyzed each complete article to determine whether it took a positive, negative, or neutral position toward the industry. For example, any article that called for restrictions or a prohibition on DTC advertising—a position that the industry opposes—was deemed negative. In contrast, an article that claimed that DTC advertising resulted in more informed patients was designated as positive.
Balance Despite the dominant position taken by the article, we also looked to see if it included the opposing viewpoint. When an explicit statement with an opposing view was included—even if it didn't receive equal coverage—we concluded that the article covered both sides. When the opposing view was not presented, the article was labeled as one-sided.
More Good News for Pharma
The Issues at Hand
There was a drastic drop in news ink on the industry in 2007, compared with the previous year. (See "Pharma Steps Out of the Spotlight," .) In 2007, 147 articles reported or editorialized on issues facing pharma. That compares with the 271 and 270 articles that ran in 2005 and 2006, respectively. (However, there was more coverage of the industry in 2007 than in 2004, when this audit began.) Since media coverage tends to be negative, the drop off in terms of number of articles is perhaps good news for the industry.
Coverage of pharma decreased across the board in the five major newspapers. (See "Clipping Service," .) The Los Angeles Times had the largest drop, running 60 percent fewer stories in 2007 than it had in 2006. While the number of front-page pharma stories was down, there was an even bigger change inside the West Coast paper: The number of editorials dropped from 36 in 2006 to 8 in 2007. The New York Times also saw a significant drop in editorials: It ran 64 articles on pharma issues in 2006 and only 22 in 2007.
For the articles that did run, what did they cover? Here's what the audit of the four factors revealed:
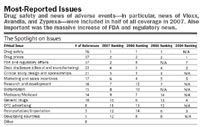
Drug safety dominates The issue of drug safety leapt into full public view in 2005, in the aftermath of the Vioxx recall. Since then, it remains the top-reported issue affecting the industry and has become increasingly prominent in the nation's conciousness. Indeed, while the number of articles on this topic had only a marginal uptick, from 73 in 2006 to 76 in 2007, the proportion of stories on drug safety leapfrogged from 27 percent of all articles in 2006 to more than half (52 percent) in 2007.
Drug prices, a mainstay It's hardly a surprise that drug prices continue to be a sensitive issue. However, what is surprising is that, in terms of coverage, it was a distant second to drug safety, with only 37 mentions. While out-of-pocket drug prices continue to be an issue for the patient/consumer, that concern is trumped by drug safety.
Most-Reported Issues
Intense FDA scrutiny The topic of FDA and regulatory affairs had the most remarkable change, catapulting from the middle of the pack last year to tie for second place (from 15 articles in 2006 to 37 in 2007). Some of these articles discussed the FDA Revitalization Act that "would give the Food and Drug Administration new power to police drug safety, order changes in drug labels, regulate advertising, and restrict the use and distribution of medicines found to pose serious risks to consumers" (New York Times, May 5, 2007).
More R&D stories, good news for pharma Another significant change was the re-emergence of articles on research and development, jumping back up to seventh on the list from its rank of 12th last year. This is generally good news for the industry, as these articles tended to present and expand the discussion around the costs and risks of drug research as well as the promise of new drug development. In the study period, there were 16 articles that focused on R&D, nine of which were positive about pharma.
Juggling places Data disclosure, clinical study design, and marketing and sales incentives used in the industry continued to attract attention, ranking four through six on the list. In particular, while there is indication that pharma is managing the sales and marketing issue more effectively through implementation of PhRMA Guidelines (dropped from No. 4 in 2006 to No. 6 in 2007), it is still a source of negative media coverage with 17 mentions this year.
New issues replace old Also attracting attention in 2007 is bioterrorism, previously included in the "Other" category. But with 15 articles in 2007, up from 12 in 2006, it is prominent enough to count as a stand-alone issue. Meanwhile, articles on generic drugs and importation/reimportation have faded in prominence since the beginning of the study.
Good News, Bad News
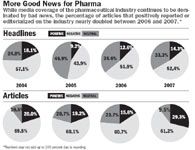
Certainly, it's no secret that the media coverage of the industry tends to be negative. But what's important to watch is a growing polarization of the reporting. (See "More Good News for Pharma," .) In 2007, there were fewer neutral articles and more negative articles—but there was an increase in the number of positive articles for pharma too.
To determine the position of the article, we analyzed headlines and the full-text body copy. Here's what we found:
Negative headlines In 2007, headlines were mostly negative (52.4 percent), inching up from 51.9 percent of articles holding anti-pharma positions in 2006 and 42.9 percent in 2005. Since headlines can be influential in shaping public perceptions and are often all consumers read or remember from their morning newspaper, these results again represent a challenge to the industry.
Positive articles double Reversing a trend, 2007 saw an increase in the number of positive articles and editorials for pharma. In fact, the proportion of positive articles almost doubled, from 15.6 percent to 29.3 percent of all articles. This could be due to more balanced reporting.

Pharma Steps Out of the Spotlight
More balance Regardless of whether articles took a primarily positive or negative tone toward the industry, we analyzed whether both sides of the disputed issue are at least acknowledged. Last year, 70.7 percent of articles mentioned both sides, up significantly from 58.5 percent of the articles in 2006. This can stem from a variety of factors on the part of the media or the industry. For example, perhaps industry execs are finally starting to weigh in on the issues of the day.
Who's News?
This year, we introduced a new analysis into the audit: We tracked pharma companies and drug brands mentioned in newspaper articles. This analysis provided an excellent crosscheck between our findings about the issues and the companies and products responsible for generating media attention related to those issues.
In the study period, the top-five newspapers mentioned 47 pharma companies. However, reporters focused most attention on 13 firms, which were mentioned five or more times. Leading the pack was Merck, which had 25 mentions on these papers' front and editorial pages. Following closely were GlaxoSmithKline, with 24 mentions, and Eli Lilly, with 16. Not surprisingly, the companies most often mentioned were linked to products most often cited in the news.
When it comes to Merck, much of the newspaper coverage continues to be driven by the legal battles concerning Vioxx (rofecoxib) and its association with increased risk of cardiovascular events. Other coverage that mentioned Merck surrounded the FDA approval of Gardasil, its vaccine that guards against cervical cancer and genital warts caused by human papillomavirus (HPV). State proposals that would mandate HPV vaccinations of young girls was the focus of many of these pieces. For example, the Washington Post reported that Virginia Governor Timothy M. Kaine would sign a bill that required shots for all sixth-grade girls unless their parents object to the inoculation (March 9, 2007).
Safety issues and news of adverse events drove other coverage of companies and brands. GlaxoSmithKline, for example, had 24 mentions, stemming mostly from the risk of heart problems associated with its diabetes blockbuster Avandia (rosiglitazone)—and Avandia-combination drugs Avandamet (rosiglitazone maleate/metformin) and Avandaryl (rosiglitazone maleate/glimepiride).
Eli Lilly came in a distant third, with 16 mentions. Eleven of these articles stemmed from its antipsychotic medicine Zyprexa (olanzapine), for the treatment of schizophrenia and bipolar disorder. Coverage was driven by the link between Zyprexa and drug-induced diabetes experienced by thousands of patients taking the medication.
Looking Forward
Due in part to remnants from Vioxx, Avandia, and Zyprexa, drug safety is once again the number-one issue attracting media attention. If drug safety is viewed more broadly—the way clinical studies are designed, the way data are reported, and how those data are reviewed by FDA—the focus on drug safety is even more pronounced (FDA would rank as the subject of 37 articles; data disclosure, 22; clinical study design, 21). Combined with the 76 times drug safety was identified as an issue, the mentions serve to reaffirm the importance of the drug safety issue in the eyes of the media—and the public.
Even a comprehensive, well-designed study cannot anticipate all possible negative coverage. In order to address this, pharma needs to manage the things it can control—particularly clinical study design and data disclosure. This becomes even more evident when one considers the cases of Vytorin (ezetimbe/simvastatin) and Trasylol (aprontinin).
We also predict that the pendulum will swing back toward more pharma stories. Media coverage may have decreased for pharma in 2007 because it shifted to Big Oil. The price at the pumps and for home heating has become a more imminent financial crisis than pharmaceuticals for many people and has, consequently, attracted related media attention. Another reason may be that 2007 was a prequel to the 2008 presidential election. The campaign tended to focus more on the war, economy, and immigration last year, but the healthcare debate is likely to emerge as key decisions about it will be made leading up to the election. Examples are the simplification of Medicare Part D and sufficient funding for children's healthcare.
This spectrum of issues reaffirms that healthcare delivery needs to be viewed from the perspective of all its components and their interconnectedness. This is consistent with PhRMA President Billy Tauzin's view that drugs are only about 10 percent of the cost of healthcare delivery. Along that line, we believe this year's study identifies some changes and invites a look at the bigger picture of US healthcare delivery.
To keep pharma in the news—the good kind—industry must continue supporting their patient-assistance programs, as well as efforts from the Health Sector Assembly, AARP, and Families USA. In doing so, companies can turn the battle toward their favor in the press—and the court of public opinion.
Stephen J. Porth is a fellow of the Arrupe Center for Business Ethics and a professor at Saint Joseph's University. He can be reached at sporth@sju.eduGeorge P. Sillup is an Arrupe Fellow and an assistant professor at Saint Joseph's University. He can be reached at sillup@sju.edu Megha Chandgothia, Cynthia Slater, Molly Porth, Courtney Scardellette, and Kim Pierret also contributed to this article.

The Misinformation Maze: Navigating Public Health in the Digital Age
March 11th 2025Jennifer Butler, chief commercial officer of Pleio, discusses misinformation's threat to public health, where patients are turning for trustworthy health information, the industry's pivot to peer-to-patient strategies to educate patients, and more.
Navigating Distrust: Pharma in the Age of Social Media
February 18th 2025Ian Baer, Founder and CEO of Sooth, discusses how the growing distrust in social media will impact industry marketing strategies and the relationships between pharmaceutical companies and the patients they aim to serve. He also explains dark social, how to combat misinformation, closing the trust gap, and more.