- Sustainability
- DE&I
- Pandemic
- Finance
- Legal
- Technology
- Regulatory
- Global
- Pricing
- Strategy
- R&D/Clinical Trials
- Opinion
- Executive Roundtable
- Sales & Marketing
- Executive Profiles
- Leadership
- Market Access
- Patient Engagement
- Supply Chain
- Industry Trends
Current Status of Biosimilars and Their Impact on Pharmacovigilance
As the market for biosimilars expands, a nuanced approach is needed to balance cost considerations with patient safety and pharmacovigilance efforts.
Philipp Hofmann, MD

The FDA states that a biosimilar is a biologic medication that is “highly similar to a biologic medication already approved by FDA—the original biologic—also called the reference product.”
A parallel can be drawn to the supermarket aisle, where one can find products closely resembling successful brands at a more accessible price point. This strategy, akin to the success story of nut nougat cream, demonstrates its efficacy with little bounds. Similarly, the pharmaceutical industry offers generic alternatives to widely recognized medications.
Here, the emphasis lies not in the taste, but in the therapeutic effect of the drugs. Whether sourced from a prominent Western pharmaceutical entity, a generic manufacturer, or an unbranded producer in regions, the active ingredients in conventional medications for conditions such as hypertension or coagulation inhibition remain identical.
For example, the ubiquitous pain reliever aspirin and its active component, acetylsalicylic acid, exemplify this consistency; however, a contentious discourse surrounds biopharmaceuticals, or biologics, and their mimetic counterparts known as biosimilars.
Table. Therapeutic Use of Biosimilars
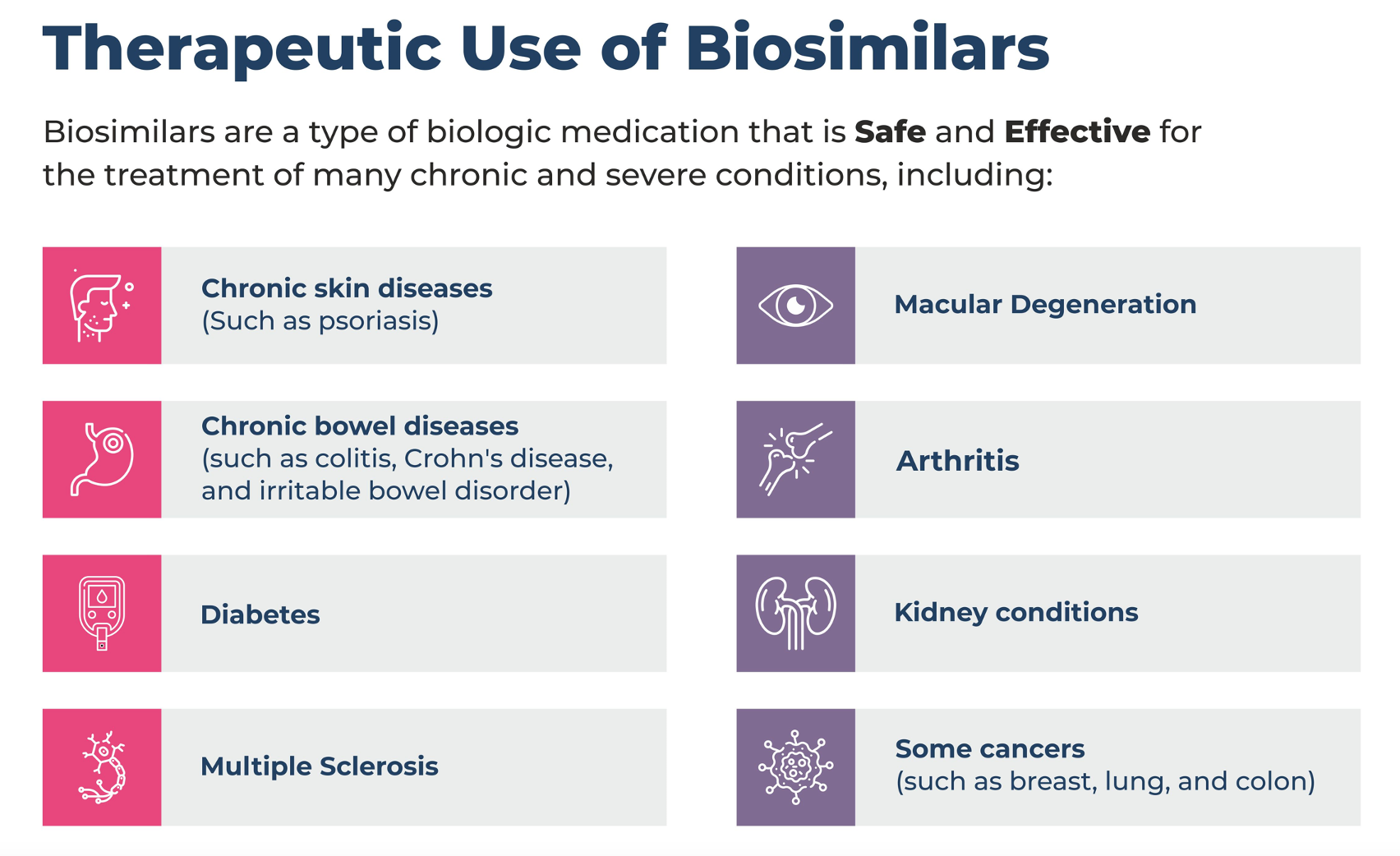
This article explores the history and implications of biosimilars, focusing on the ongoing discussions surrounding their interchangeability with biologics and the potential effects on pharmacovigilance.
Reflecting on the History of the Biopharmaceutical Industry
The introduction of biopharmaceuticals occurred four decades ago, leading to the approval of more than 200 biopharmaceutical substances. These substances encompass a wide range of applications, from hormones and vaccines to treatments for cancer, autoimmune diseases, and coagulation disorders.
While biologics have demonstrated remarkable efficacy, they come at a considerable cost, with some therapies amounting to up to 25,000 euros per year per patient. Given that individuals with conditions such as diabetes, rheumatism, or cancer often require extended or lifelong treatment, demand for more affordable alternatives is substantial.
As patent protections wane, biosimilars have entered the market, with the first of these becoming available in 2006. Currently, there are 86 approved biosimilars for 16 biologic active ingredients in European markets, offering an average cost reduction of approximately 30% compared to their original counterparts. However, pricing remains relatively stable due to the lengthy and expensive development process of biosimilars, which takes between eight to ten years.
Cost efficiency could be one of the reasons for the continued growth of this industry. The projected growth of the global biosimilars market indicates an increase from nearly $30 billion in 2023 to nearly $70 billion by 2028, reflecting a compound annual growth rate of 18.32% over the forecast period of 2023-2028.
Comparing Biosimilars to the Original Biologics
Before receiving marketing authorization, biosimilars undergo rigorous testing to ensure their efficacy and safety are comparable to the original biologic. The European Medicines Agency (EMA) affirmed in a statement from September 2022 that this exchange is safe within these established criteria.
Table. Biosimilars and Biologics
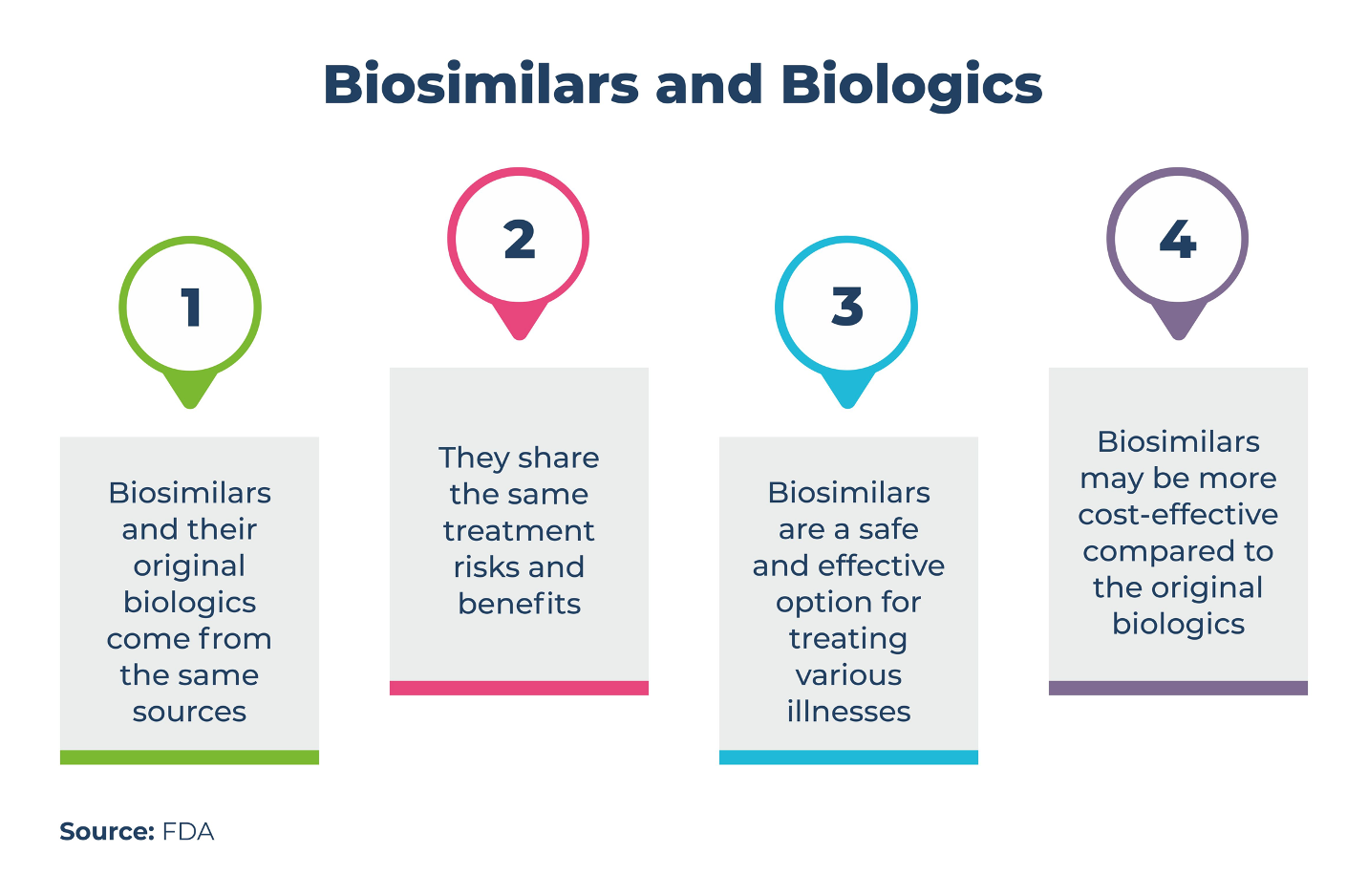
Nonetheless, it is important to acknowledge that although biosimilars share identical amino acid sequences and effects with their original counterparts, they may vary in minute details due to the production process involving microorganisms or cell cultures. These distinctions may necessitate specialized analyses for identification.
Challenges in Adoption and Impact on Pharmacovigilance
The push for enforced substitution of biologics with biosimilars has ignited a contentious debate. Health insurance providers advocate for the use of the most cost-effective biosimilar, projecting potential savings of 750+ million euros in the German market in 2019. However, healthcare professionals, patients, and manufacturers oppose automatic substitution due to the potential for adverse drug reactions (ADRs), such as allergic reactions or intolerances. In such cases, healthcare providers may not be aware of the specific preparation dispensed by pharmacists.
Pharmacovigilance Considerations
For both innovator biologics and biosimilars, robust pharmacovigilance systems are crucial to address the complexity of safety data and challenges in detecting ADRs. The interchangeability of biosimilars further complicates matters, as one biosimilar may be used in place of another referencing the same product. Reporting of ADRs for biologics varies by region, with the European Union mandating the inclusion of brand names and batch numbers.
The debate surrounding biosimilars continues to shape the pharmaceutical landscape, with legislative changes anticipated. As the market for biosimilars expands, a nuanced approach is needed to balance cost considerations with patient safety and pharmacovigilance efforts.
Works Cited
- Statement on the scientific rationale supporting interchangeability of biosimilar medicines in the EU. European Medicines Agency. News release. April 21, 2023. https://www.ema.europa.eu/en/documents/public-statement/statement-scientific-rationale-supporting-interchangeability-biosimilar-medicines-eu_en.pdf. Accessed February 7, 2024.
- https://aok-bv.de/presse/pressemitteilungen/2019/index_21662.html
- Interchangeability of Biosimilars. Federal Institute for Drugs and Medical Devices. News release. September 29, 2022. Accessed February 7, 2024. https://www.bfarm.de/EN/Medicinal-products/Licensing/Types-of-marketing-authorisation/Interchangeability-of-Biosimilars/_artikel.html
- B Oza, S Radhakrishna, P Pipalava, and V Jose. Pharmacovigilance of biosimilars – Why is it different from generics and innovator biologics? J Postgrad Med. 2019 Oct-Dec; 65(4): 227–232.
- Ferreri, D. Why Pharmacovigilance Is Important for Biosimilars. The Center for Biosimilars. Published March 31, 2020. Accessed February 7, 2024. https://www.centerforbiosimilars.com/view/why-pharmacovigilance-is-important-for-biosimilars
- Biosimilars Market Size & Share Analysis - Growth Trends & Forecasts (2024 - 2029). Mordor Intelligence. https://www.mordorintelligence.com/industry-reports/global-biosimilars-market-industry. Accessed February 7, 2024.
About the Author
Philipp Hofmann, MD, started his career as a medical writer in the pharmaceutical industry. This first position was soon followed by a continuous professional development from Medical Manager, Director Global Safety to Head of Pharmacovigilance and EU QPPV. Philipp, with his specialization in Pharmaceutical Medicine, is now Director Pharmacovigilance & Global QPPV, Navitas Life Sciences.
Fighting the Drop in Vaccination Rates: Q&A with Gregg Sylvester
July 26th 2024An unexpected consequence of the COVID 19 pandemic has been a loss in trust of vaccines. Over the past three flu seasons, there has been a drop in vaccination rates that is alarming experts. Dr. Gregg Sylvester, chief health officer at CSL Seqirus, spoke with Pharmaceutical Executive about the causes of the decline and how important it is to get the rates back to normal.
Cell and Gene Therapy Check-in 2024
January 18th 2024Fran Gregory, VP of Emerging Therapies, Cardinal Health discusses her career, how both CAR-T therapies and personalization have been gaining momentum and what kind of progress we expect to see from them, some of the biggest hurdles facing their section of the industry, the importance of patient advocacy and so much more.
