
Pharmaceutical Executive
Industry self-policing may be the only way to stop state Legislators who want to ban sales Reps, and even whole companies, from using Prescribing information.

Pharmaceutical Executive
Industry self-policing may be the only way to stop state Legislators who want to ban sales Reps, and even whole companies, from using Prescribing information.
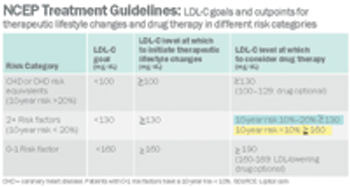
Pharmaceutical Executive
The world's largest drug manufacturer must answer off-label promotion charges brought by a new adversary. Not FDA, with its warning letters and threats of marketing sanctions, and not the Office of the Inspector General (OIG) at Health and Human Services, which often sues for fraud, forces huge settlements, and requires companies to do business under restrictive corporate integrity agreements. Instead, the company faces a class-action civil suit from insurance companies and union welfare funds, groups that, until recently, Pfizer regarded primarily as customers-or at least people who picked up the tab for customers. Now, led by the Welfare Fund of a Teamsters local from New Jersey, third-party payers are suing under RICO, the Racketeering Influenced and Corrupt Organizations Act. If their suit is successful, payers who have covered billions of dollars worth of Lipitor (atorvastatin) over the past five years will receive treble damages for the cost of off-label prescriptions. The suit may also attract..

Pharmaceutical Executive
Are large corporations, with massive bureaucracies and thousands of employees, capable of acknowledging employees' personal passions? They don't have a choice. Large numbers of talented people are leaving organizations because the corporate structure is not accommodating personal passion. The good news is that the great companies are starting to do something about it, which denotes a big change in business ideology. Bill Toppeta, president of MetLife International, recently told the Fordham Leadership Forum, at the Fordham Graduate School of Business, "What you need to know as the leader is what motivates your people, not what motivates you."

Pharmaceutical Executive
Criminal penalties for violating the Foreign Corrupt Practices Act can be substantial. Businesses found guilty may be fined upwards of $2.5 million for each offense, or twice the amount gained as a result of the violation.

Pharmaceutical Executive
The pharmaceutical companies in the Predictive Safety Consortium have agreed to cross-test each other's laboratory methods to determine which are most effective in detecting kidney, muscle, and liver toxicity.

Pharmaceutical Executive
Where is pharma going? Toward more science, and more political pressure on science. Toward greater patient responsibility-and more regulation-by-lawsuit. And forward. Let's not forget about forward.
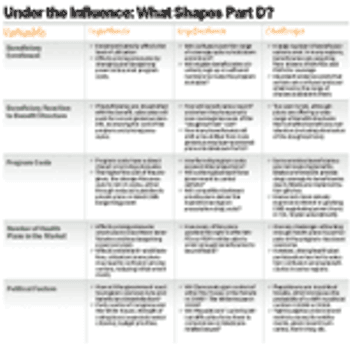
Pharmaceutical Executive
As seniors become frustrated and Part D enrollment lags, drug benefit plans cannot grow fast enough to manage risk. Some leave the market, and others cut benefits.

Pharmaceutical Executive
The investigators, other than the people at Merck, didn't know about [additional cardiovascular adverse events] for six months after the study was published. But one could argue we didn't have to know because it's not part of the predefined study.

Pharmaceutical Executive
I've just returned from Europe, where I spoke with various think tanks, thought leaders, and pharmaceutical companies on the issue they call "information-to-patients" (ItP). That's what we on the other side of the pond refer to as direct-to-consumer communications. In Europe, they choose to abstain from using the "A" word-advertising. But rhetoric counts. "Information to patients" seems quite paternalistic when compared with the new-worldly "direct-to-consumer" moniker.

Pharmaceutical Executive
Changing Landscapes: A Special Report on the World?s Top 50 Pharma Companies

Pharmaceutical Executive
One health insurance company in The Netherlands is offering doctors a financial incentive to prescribe generic statins and proton pump inhibitors. Doctors and patients complained, but a court upheld the practice.

Pharmaceutical Executive
Just how traditional marriages join a couple together from a common culture, Daiichi and Sankyo are merging based on a sense of having come from the same place, and facing the same future. But the art of integration lies in creating new ways of working that make the marriage bigger than the sum of its parts. Officiating the marriage is John Alexander, MD, head of pharma development for Daiichi Sankyo.

Pharmaceutical Executive
The Zelnorm study was conducted for $1,000 per patient, a fraction of the price of clinical trials, which can cost $10,000 per patient or more.

Pharmaceutical Executive
Mergers have cut the field of companies with real marketing and manufacturing muscle from 25 to five. The 2004 vaccine market will double by 2009.