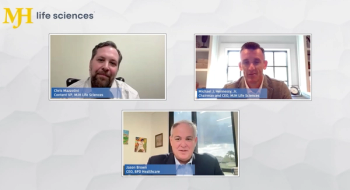
In this video discussion with Pharmaceutical Executive, Michael J. Hennessy Jr., chairman and CEO of MJH Life Sciences, and Jason Brown, CEO of BPD Healthcare, discuss how the deal came together and what it means for healthcare executives navigating an increasingly complex marketplace.