
All News


Critics of the pharma industry have gotten good at selective reporting. So good, I've started tuning out. But, with a public official pulling similar punches, my ears can't help but perk up.

June 06 BPA Statement

Merck's Januvia has a head start, but Novartis isn't sitting idle.

An executive interview with Lee Babiss PhD, VP, Preclinical R & D of Hoffmann-La Roche, Inc, conducted by Patrick Clinton, Editor in Chief of Pharmaceutical Executive. Tune in and hear a real-world perspective on drug repositioning as a new way of filling late-stage pipelines.

Drug giant beefs up early-stage R&D, moving into Genentech's turf.

After axing oncology, Gilead says it's ready for new therapeutic areas.

Pharmacists have applauded Plan B's behind-the-counter status, and FDA hints that the drug might pave the way for more pharmacy-only OTC products.

A new, faster recipe for drug development: Chat up regulators. Outsource where you can. And know when to let go.

The recent settlement between branded and generic companies offers a 'win-win' approach to patent challenges.

Drug makers used aggressive contracting to retain marketshare after launch of generic simvastatin

Pricing, marketing cases expected to escalate in the future

Pharmaceutical Representative is pleased is pleased to bring you informative, interactive, and cost-effective web seminars. These time-saving events are user friendly and designed to deliver information that will improve your bottom line. Please review the list below for upcoming topics.
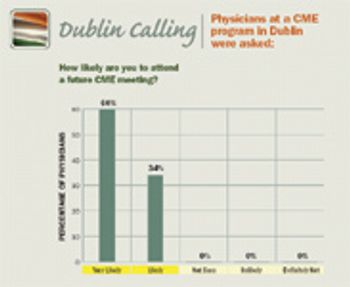
It's clear CME is becoming an important part of global healthcare, and there's no better time for pharma companies to capitalize on this growing trend. There's enormous opportunity, but it must be tailored to the distinct clinical environment of each country.

Company faces two Vioxx setbacks in court as it prepares to reveal safety data on its cox-2 successor.

Recent analysis shows partial or complete invalidation of all pharma patents challenged since December 2004.

Schering-Plough's successful allergy medication gets an updated ad campaign.

Revolutionizing Pharmaceutical Marketing Effectiveness with Change Management and Technology

Revolutionizing Pharmaceutical Marketing Effectiveness with Change Management and Technology

Bioterrorism and larger profit margins could drive future growth.

Biotechs find sales more profitable than IPOs.

Partnership with Momenta includes technology to evaluate bio-equivalence.

Online advertising opportunities

Think you've been around a long time? When's the last time your company gave a doctor a cigarette lighter with your logo on it? Put any corks in medicine bottles lately? Can you remember the last hero shot of a pharma CEO on the cover of Time? We can. And we have the artifacts to prove it...
Is the golden age of drug development over? Fat chance. Decade-by-decade comparisons show how new medicines improve on old ones. And that's innovation.

Pharma restrictions of sales tactics vary from state to state, creating new compliance challenges.

The road forward for both public health and the industry is going to require more trust and intimacy, not less, between patients, physicians, FDA, and pharma. Patients need to see a fully functioning set of checks and balances-not what they're seeing today.

Pharma leaders (and Pharm Exec readers) reflect on the ideas and events that revolutionized the industry over the last quarter of a century--and what to watch for in the next 25.

See how well you did on Pharmaceutical Executive's 25th anniversary quiz.