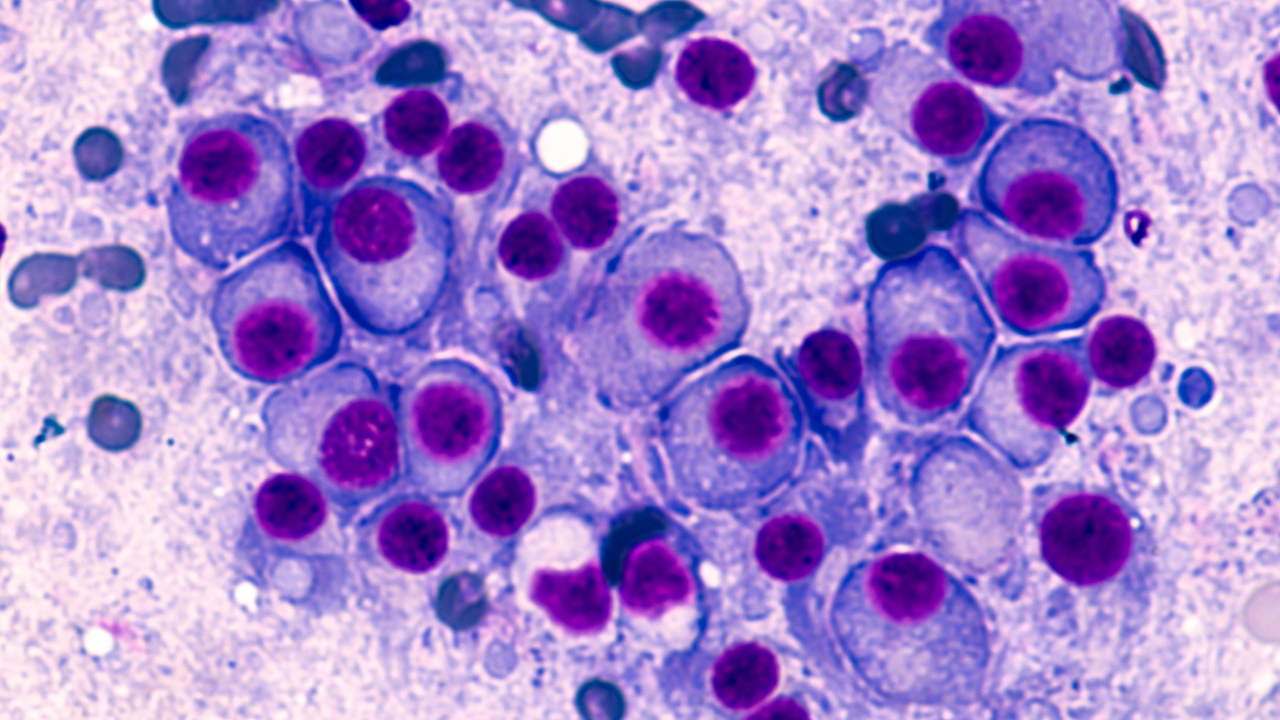
In an interview with Pharm Exec Associate Editor Don Tracy, Pedro Valencia, VP, Solid Tumor Pipeline Strategy & Execution, AbbVie, discusses how AbbVie is advancing the development of bispecific biologic therapies in both solid tumors and blood cancers.