
Successful innovation now has to align with key metrics of value-can an old baseball metaphor help guide the way?

Successful innovation now has to align with key metrics of value-can an old baseball metaphor help guide the way?

Where are the biosimilars to help cut costs as the first wave of biologics, or complex, small molecule respiratory drugs, for example, go off patent? They've arrived in Europe, but the U.S. lags...

If R&D doesn't rebound, the growth prospects of R&D services providers will suffer, and even commercial CMOs will see fewer opportunities coming their way, writes Jim Miller.

Real-world pharmacoeconomic data can provide critical insight for maximizing US market access and oncology product differentiation, and should be central to the overall brand strategy of any oncology product

Industry experts talk to Pharmaceutical Technology's Adeline Siew about the requirements and challenges involved in getting a biosimilar product to market.

Companies are leveraging combinations of drugs and other products to gain competitive advantage and market share.

With their knowledge of molecular genetics, Pathologists are transforming the way healthcare is provided.

The lifeblood of the life sciences industry is continuous innovation-it is not a business that can survive by standing still.

Project Data Sphere aims to liberate clinical trial data sets from industry and academic vaults, in an attempt to catalyze cancer research and discovery.

While not as bleak as believed, the outlook of European biotech sector is struggling compared with its US counterpart.

New research from the Tufts Center for the Study of Drug Development identifies a significant contributor to the rising cost of clinical trials-the first step in meeting a growing strategic imperative to help senior management and the regulatory community craft new approaches to make trials more efficient in delivering results to clinicians and patients.

Improving the quality of clinical trials is a major strategic activity for Big Pharma, one where efficient management of a demanding set of regulatory requirements can have a positive impact on the bottom line.

Pharm Exec recently convened a cross-section of industry experts to review current and pending efforts to turn great science into good oncology practice.


With the shadow cast by Mediator across Europe now receding to a focus on the French law courts, the European Union (EU) said in late November that its pharmacovigilance arrangements offer “one of the most advanced and comprehensive systems in the world”.

The Patient-Centered Outcomes Research Institute, or PCORI, announced last week that it will be granting a total of $12 million for up to 14 contracts for studies aimed at improving upon existing research methodologies to demonstrate clinical effectiveness.
Ideology, politics, and a stilted political debate may be causing pharma to overlook the potential of emerging stem cell therapies in fostering a new generation of cures.

There is much talk today about "open innovation" in business and research forums-but what exactly does it mean? How does open innovation as a concept apply to the pharmaceutical sector? Does it signal a change in the way pharmaceutical companies approach research and innovation?
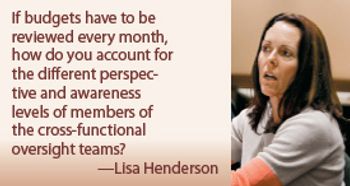
Mastery of the clinical trial process has become essential to positioning new therapies for leadership in an increasingly crowded-and lengthy-race to registration.

As brands are required to produce more and more data to convince not only regulators, but payers, physicians, and patients, Jeffrey Jonas is pushing Shire's "search and develop" R&D model into new and sometimes uncomfortable territory.

Poland's government is aiming to make the Eastern European country a biotech powerhouse.

To gain market access in emerging markets, pharma companies face pressure to offer a convincing Corporate Value Proposition to governments.

After his predecessor and countryman left the EMA in disgrace, Guido Rasi jumps in as executive director-with great challenges ahead.

Sponsor sourcing requirements are diverging from the operating needs and long-term strategies of the major CROs.

A new generation of products for inflammatory disease is coming forward, promising better promise and possibly a cure.