- Sustainability
- DE&I
- Pandemic
- Finance
- Legal
- Technology
- Regulatory
- Global
- Pricing
- Strategy
- R&D/Clinical Trials
- Opinion
- Executive Roundtable
- Sales & Marketing
- Executive Profiles
- Leadership
- Market Access
- Patient Engagement
- Supply Chain
- Industry Trends
Say Hello to Flexible: Reimagining the Patient Journey
Through embracing connected and tech-enabled services and devices, new avenues and opportunities exist for greater clinical trial flexibility in drug research.
Adam Halbridge, Clinical Trial Tokenization Lead and Executive Director, ICON plc

When we think of the word flexibility in the context of research, we should take into consideration that this is a departure from the longstanding “rigidity” in the way drug research has traditionally operated. Clinical trial flexibility means getting creative, growing strategic partnerships, and potentially changing traditional patient behaviors to new behaviors that ultimately serve them better. Flexibility is reimagining the patient journey and rebalancing the workload for each stakeholder involved.
A powerful example of this juxtaposition of flexible versus rigid can be seen in how clinical research sites currently operate. Clinicians are more empowered—thanks to patient platforms and connected devices. They also have a clearer picture of the individual’s journey thanks to tokenization capabilities. In-home visits are reducing research workload and reducing burden on the participating individuals. When we implement fit-for-purpose technologies and services—that include out-of-the-box technology solutions and/or bespoke services and technology architectures—we see an even greater shift in value. In fact, recent analysis shows that if it is assumed that connected methods are applied to both Phase II and Phase III trials, the increase in value is $20 million per drug that enters Phase II, with a seven-fold ROI.1
As a result of these measures, the drug development industry should be able to elegantly implement and articulate how the individual, who we often regard as the patient, moves through their respective research or healthcare journey. That insight is hugely important.
Comprehensive data assets (i.e., real-world evidence), artificial intelligence (AI), machine learning (ML), and enhanced evidence through clinical trial tokenization are the new baseline components required for successful asset development and patient safety. In our experience, it is the integration of these data assets and technology combined with strategic and supportive services that will really shift the dial on drug development and support the flexibility and creativity that benefit the patient’s journey. The tools that are available in this regard include:
- Predictive modeling for recruitment and retention
- Optimizing trial design
- Safety and efficacy indicators
- Unknown drug-drug interactions
- Clinical trial tokenization
- Measuring the likelihood of post-marketing commitment risk
- Digital risk detection
TOKENIZATION IN CLINICAL TRIALS
Tokenization represents a new and powerful avenue that supports the research, development, and approval process for new therapeutic products. There has been a lot of confusion around tokenization regarding patients and participants in research and the tokenization of commercial or real-world data. When we tokenize a trial patient, there are unique FDA-imposed requirements that must be followed to maintain compliance. As a case in point, for the past five years, our clinical trial tokenization process at ICON has undergone regularly scheduled internal and external expert determinations of our validated process to maintain FDA and good clinical practice compliance.
Tokenization begins with the informed consent process either in a document form or through an eConsent process (see Figure 1 below). During this step, the individual is requested to consent to their study data being used for secondary research.
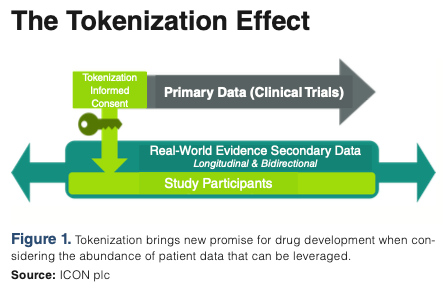
When the individual or patient has consented, certain details are submitted to the tokenization system such as name, gender, date of birth, zip code, and study details. The patient identity changes from “Jane Doe” to a string of characters such as: 5678913654782679853497659. Over time, the token can be matched with real world de-identified datasets like Symphony’s Integrated Dataverse®뿣, which is one of the world’s largest repositories of healthcare data and an ICON company.
Standard and advanced aggregate analytics provide insights relating to demographics, comorbidities, and concomitant medicines. Tokenization brings new potential for clinical development when we consider the immense volume of patient data that can be leveraged and added to the clinical trial data through this process.
ADAPTIVE STUDY DESIGN
Flexibility in study design is yet another powerful construct that can shift the patient journey in real-time based on data and analytics. The flexibility we are focused on is grounded in these advanced approaches that save time, money, and increase efficiencies across the asset’s development lifecycle.
Accelerating drug and device development
Costly data-collection methods, widely used today, are getting recognized as cost centers, and a poor use of human capital when this process can otherwise benefit from automation.
Our experience is that eClinical tools, which includes e-sourcing data, can, in some instances, be informed through AI. Electronic data capture (EDC) has taken us far beyond what EDC meant to us five years ago and is reshaping the process of evidence-generation. Comprehensive, clean data reduces the burden associated with data management across various stakeholders. Flexibility to embrace these powerful tools provides researchers with a more complete view of the patient’s state and provides a deeper understanding of possible patient outcomes.
Furthermore, remote patient monitoring not only enhances patient convenience but also increases the geographical reach of clinical trials. Participants who may have been excluded due to location constraints or mobility issues can now actively contribute to research, leading to more diverse and representative study populations. This democratization of participation is a key aspect of the greater trial flexibility facilitated by technology-enabled services.
The internet-of-things (IoTs) Impact on remote patient monitoring
IoT, through a growing universe of devices such as wearables and sensors, has propelled the concept of remote patient monitoring to new heights. Wearable devices collect continuous, real-time data, providing a wealth of information that transcends periodic site visits. This not only reduces the logistical challenges faced by patients, but also broadens the geographical reach of clinical trials and provides fast source data. This accessibility and granularity contribute significantly to trial flexibility, as patients become active partners in the research process.
AI and enhanced analytics
The infusion of AI in clinical research extends beyond trial design and patient monitoring. ML algorithms are revolutionizing data analysis, interpretation, and decision-making. AI processes vast datasets at unprecedented speeds, uncovering patterns and correlations that were previously hidden.
In predictive analytics, AI aids in identifying potential risks and opportunities, contributing to more informed decision-making by researchers and sponsors. Moreover, AI-driven algorithms can assess patient responses to treatments, identifying nuanced patterns that may escape manual analysis. This analytical prowess enhances the overall efficiency of clinical trials, ensuring that researchers can adapt protocols swiftly based on real-time insights.
Provenance in research data and analytics: Blockchain
The adoption of blockchain technology is addressing critical challenges related to data integrity, chain of custody, and security. Blockchain ensures that data collected during clinical trials cannot be tampered and is verifiable, reducing the risk of fraud and enhancing the overall trustworthiness of study results.
DIGITAL HARMONY
The symphony of technology and data assets are powerful tools. Importantly, when these are combined with the expertise of experienced delivery operations teams, more predictable outcomes can be generated. These are tools that, encouragingly, are being increasingly rolled out by drug-development stakeholders across the industry—a welcome development that emerged from the COVID-19 pandemic.
Modernized digital research is at our doorstep and it is reducing burden across the board. We know adoption and learning challenges exist, but if the research community is willing to work collaboratively along this road to modernization, healthcare emerges the winner and patients will reap the benefits.
Adam Halbridge, Clinical Trial Tokenization Lead and Executive Director, ICON plc
Reference
1. DiMasi, J.A.; Smith, Z.; Oakley-Girvan, I. Assessing the Financial Value of Decentralized Clinical Trials. Ther Innov Regul Sci. 2023. 57 (2), 209-219. https://pubmed.ncbi.nlm.nih.gov/36104654/

Cell and Gene Therapy Check-in 2024
January 18th 2024Fran Gregory, VP of Emerging Therapies, Cardinal Health discusses her career, how both CAR-T therapies and personalization have been gaining momentum and what kind of progress we expect to see from them, some of the biggest hurdles facing their section of the industry, the importance of patient advocacy and so much more.
