
Jin Zhang provides an overview of China's major bispecific antibody developers.

Jin Zhang provides an overview of China's major bispecific antibody developers.

Disparities in income levels in China continue to drive far-reaching differences in how poorer and richer cancer patients are treated, with recent data suggesting that the differences in access to treatment lead to different outcomes for poorer patients. Peter De Richter reports.

What will come out of Austria’s shakeup of European pharma rules?

The call to reduce market entry times for life-saving new drug therapies is becoming more urgent. What constitutes best practice in managing translations in today’s regulatory affairs environment? Nancy Pollini reports.
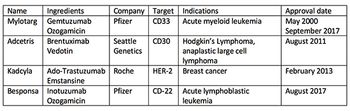
In the face of booming antibody market, China has jumped into the antibody-drug conjugate space. Jin Zhang reports.

Tyrosine-kinase inhibitors (TKIs) are gradually becoming the most popular option for lung cancer treatment in China. Jin Zhang reports.

A recently announced collaboration between EUnetHTA, WHO, and ISPOR brings home the fact that there is still no real agreement on a definition of HTA, writes Reflector.

Maude Schmidt and Giovanni Saldutti look at the intergovernmental initiatives involving joint price negotiations developed across Europe as the Beneluxa Initiative on Pharmaceutical Policy to explore the outcomes and implications from these alliances.

Record capital flows across global public and private markets has opened up new channels of investment in early-stage and novel science.

In an attempt to reduce high drug prices and improve its medical insurance coverage, China has initiated a series of new moves, including the recent zero import tax on a range of anticancer drugs. Jin Zhang reports.

Over the last decade, Spain has seen a significant growth in venture-capital investment in the life sciences. We explore the driving forces.

Research-based companies in Europe look as though they have lost one battle on preserving incentives for innovation – but the bigger war is only now getting underway, writes Reflector.



Artificial intelligence adoption by pharma companies in the UK demands a new, agile governance layer, write Tim Wright and Antony Bott.
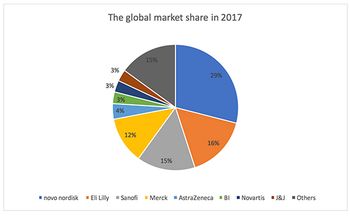
With China predicted to account for 48% of the global diabetes population by 2045, Jin Zhang looks at how the country's domestic pharma companies are faring in this treatment area.

Global pharma companies and Japan can create a win-win situation through a new pricing mechanism, writes Nobuko Kobayashi.

Raman Sehgal talks to three industry leaders for their insights on the challenges faced across the life science sector and how the EMA needs to respond.
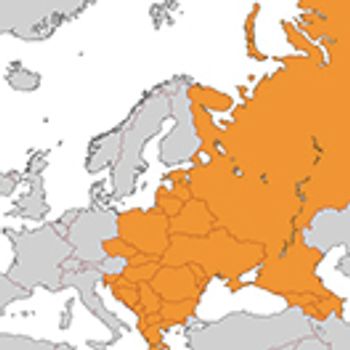
Filip Conic outlines the market-access approaches and commercial strategies to unlock the growth potential in the Southeastern and Eastern European region.

Exploring the prospects-and related challenges-for Chinese life science as it aggressively pursues new growth areas.

New Jersey is still a top pick for companies when it comes to pharma.

Korean biotech opens first U.S. headquarters in New Jersey to better control clinical trials as it looks for strategic partnerships in the U.S.

Enzychem Lifesciences explains why the Korean biotech decided to open U.S. headquarters, as well as gives an update on their most recent clinical trials.

China's nurse educator program aims to improve quality of care and make life simpler for physicians handling high patient load. Sebastian Bather reports.

Figuring out the workings of the EU in topics related to health can be akin to deciphering Egyptian hieroglyphs, making it challenging for companies to plan ahead, writes Reflector.