
The Innovation Scorecard was developed to track compliance with NICE Technology Appraisals. Leela Barham reviews its latest update.

The Innovation Scorecard was developed to track compliance with NICE Technology Appraisals. Leela Barham reviews its latest update.



Nine months on from the 2016 EFPIA Disclosure Code deadline - requiring all member companies across Europe to publish their data concerning their transfer-of-value transactions to HCPs - EFPIA's Andrew Powrie-Smith offered an update on media and industry responses.

The EMA released the revised Module V- Risk Management Systems (Rev 2) of Good Pharmacovigilance Practice (GVP) (EMA/838713/2011 Rev 2) accompanied by a revised Guidance on the format of the risk management plan (RMP) in the EU.

Is the Greek pharmaceuticals and healthcare sector, once a strong bridge between Europe and the East, on the mend after suffering the recent ravages of the global financial crisis and subsequent austerity policies?


March 28, 2017



While it is foundational to commercial operations, most European life sciences companies are not getting what they need from their customer data, writes Guillame Roussel.

March 20, 2017
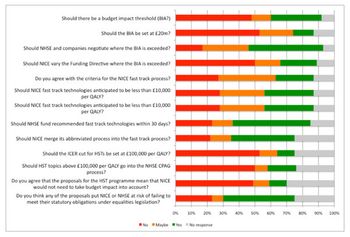
NICE and NHSE have been grappling with the issue of affordability. It’s not a new challenge, but with the advent of new treatments that are both cost effective and unaffordable, something has to be done. Leela Barham reports.

Pharm Exec talks to Hugo Fry, Sanofi’s General Manager for the UK and General Manager for Sanofi Pasteur UK and Ireland, about his vision for the company and the vaccine space in these markets.

The European Commission's latest economic assessment of EU member states does not make for comfortable reading, writes Reflector.

Pharm Exec speaks to Chris Molloy, CEO of the newly established Medicines Discovery Catapult, which aims to establish new approaches to grow and sustain the UK's reputation for innovative, fast-to-patient drug discovery.


Leela Barham looks at NICE's new position statement, which seeks to set out a little more clearly how it works with industry.


Pharm Exec convenes a panel of biopharma executives responsible for the Latin America business to discuss investment, market access, and reimbursement issues in this key and challengingly diverse growth market for the life sciences industry

February 28, 2017

With a population of just 4.7 million and a landmass smaller than the state of Indiana, the republic of Ireland stands as an unlikely pharmaceutical manufacturing powerhouse.

February 23, 2017

February 22, 2017

February 22, 2017