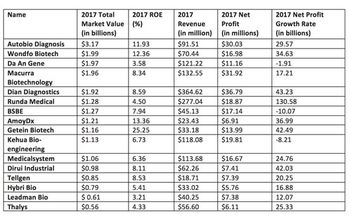
Jin Zhang identifies the major domestic companies in China’s IVD industry.

Jin Zhang identifies the major domestic companies in China’s IVD industry.

Jin Zhang discusses the research and development of several major biologics in China.

Jin Zhang looks at how China's CART-T therapy race is heating up.

Looking back at the agenda of a 1985 EFPIA conference demonstrates that, three decades on, the European drug industry is still seeking a pill for its own ills.

Outlining the four major areas driving China’s life sciences industry, which may be on the cusp of a new era in R&D and healthcare reform.

Looking back at the agenda of a 1985 EFPIA conference, Reflector sees that, three decades on, the European drug industry is still seeking a pill for its own ills.

Amid contrasting views on the success of the now well-established system of outcomes-based agreements in Italy, further demands on global healthcare budgets will likely point to their adoption on a wider scale

Pharm Exec speaks to James Burt, Accord Healthcare’s executive vice president, Europe and MENA, about the company’s vision for the UK and Europe post-Brexit, the challenges of retaining the company’s culture amid major growth, and the current landscape for generic medicines.

Jin Zhang outlines four key areas that impacted Chinese pharma in 2017 and looks at how their effects will shape the industry in the years ahead.

Scope of supplementary protection certificate (SPC) could change.

In the coming weeks, the EU will decide if the degree of protection offered to drug firms by research incentives such as the SPC are really in the interests of patients and society. Reflector reports.

Jin Zhang reviews the current fortunes of the Chinese pharmaceutical companies.

Professor Carole Longson's move from NICE to the ABPI is another appointment that helps to square the balance between UK industry and Government. Leela Barham reports.

The UK's NICE now has the job of both being a member of the newly named Accelerated Access Collaborative (AAC) and also acting as its Secretariat.

As the Ukranian healthcare market opens up to more public and private competition, it will become more attractive for investors - both Ukrainian and foreign, write Lana Sinichkina and Anna Zorya.

David Simon outlines the procedures to manage anti-corruption risk that life sciences companies should consider before doing business in China.

Reflector reports on the EU's legislative move to push the fifty or so national and regional HTA organizations in Europe towards greater cooperation.

Rachel Howard makes the case for pharmaceutical companies to include the developing world front and centre in their vision.

Is getting more clarity on Brexit ramifications for pharma and European public health really at the top of new-year to-do lists?

Pharm Exec details the latest approaches to combating disease and health crises on a global scale-including industry perspectives from those involved on the front lines.

Amid a widening net of health threats worldwide, alliances forged in research and response may be more critical than ever to stem the tide.

New action plan urges tighter alignment of public health policy with the development of targeted therapies.

The Chinese government's “Healthy China” plan presents an unprecedented opportunity for the US healthcare industry and its investors, writes Steven Shill.


With its convergence of healthcare talent and tech, the nation is turning into a prime destination for innovative startups.