
As pharma continues to move towards "beyond the pill" strategies, better understanding of patients' sometimes paradoxical view of disease can help lead to strategies for helping them live more productive lives, writes Nick Hicks.

As pharma continues to move towards "beyond the pill" strategies, better understanding of patients' sometimes paradoxical view of disease can help lead to strategies for helping them live more productive lives, writes Nick Hicks.

Nick Hicks looks at the challenges of devising a patient advocacy strategy amid the complex political, cultural and regulatory landscape of Russia and CIS.
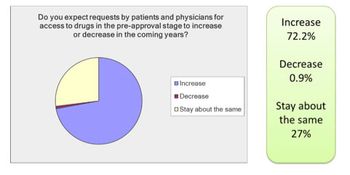
While the initiation and management of an access program can be challenging and requires careful assessment and planning, the benefits to patients, healthcare providers and sponsoring companies are immeasurable, writes Rob Fox. “Patients will find you.”In his keynote address at this year’s World Orphan Drug Congress, Henri Termeer, the former CEO, chairman and president of Genzyme reminded the audience that patients will actively seek out companies developing new therapeutics for their unmet medical needs.

Patient centricity requires clinical trials to be based on life outside the lab, writes Mads Holme.

Pharma’s image is still suffering despite a rise in medical innovation. Dan Bobear offers five patient-centered strategies that could help in finally mending the gap.

Emerging technology partnerships in the biopharma space may be provide hope in accelerating patient enrollment, writes Michael Christel.

Loreen M. Brown discusses how technology has influenced how healthcare teams function, from leveraging and delivering care to interacting with patients throughout the patient journey.

Casy McDonald looks forward to CBI's Patient Adherence and Access Summit in Philadelphia.

Gilead outlines its key access initiatives for its hepatitis C franchise.

Demands from patient advocacy groups for broader subgroup representation in clinical trials has generated a new drug trial transparency initiative at the Center for Drug Evaluation and Research (CDER).

Colleen Tracy James and Neil DuChez ask, is biosimilar litigation the new frontier as predicted or an area pursued less vigorously as under the Hatch-Waxman Act because of cost and manufacturing obstacles?

Steve Smith provides an advocates account of last month's Rare Disease Week on Capitol Hill.

Personalized medicine has reached a crossroads where fundamental change is not only appealing but essential, write Klarissa Hoday, Francesca Boggio, and Aleksander Ruzicic.

Research advocate, "cancer warrior" and survivor Jack Whelan talks to Pharm Exec about what pharma how pharma could improve its communication with patients.

Pharma jumps on board personal genomics train-at least for test drive-but will journey ultimately help transform treatment or stall out as just another fad?

Dr. Pamela Walker asks, in an increasingly competitive marketplace, with greater understanding of behavioral biases such as loss aversion, how can pharma professionals use loss aversion to propel brands to success?

Non-adherence, in the US alone, is a $100-billion-a-year problem. Healthcare players are touting patient education and engagement as the keys to better adherence rates. Ben Comer reports.

Elderly patients are underrepresented in clinical trials, but there are few obstacles to including this patient group in trials that cannot be overcome, writes Sydney Rubin.

Advocacy organizations and individual patients are getting more involved in every facet of the healthcare system, from drug R&D, to federal and state policy all fueled by the hour-to-hour passion of living with a disease.

A patient-centric approach to drug development delivers the benefits that actually create value, writes Charlotte Vansgaard and Mikkel Brok-Kristensen.

New drug delivery mechanisms and devices are an opportunity to build relationship with the ultimate customer: the patient.

In the world of patient education, processes, policies, and oversight differ vastly. There is no overarching governance structure. There isn't an accreditation process for educational interventions or patient advocacy organizations that develop the education.

Patients in search of face time with a healthcare provider are stopping into their local drugstores, where consultations are free and the wait time is shorter.

Brad Sitler looks at the challenge for pharma of engaging patients and helping physicians provide them with the right information at the right time.

Specialized knowledge about disease and treatment is no longer the exclusive province of practicing physicians. Patient voices are increasing in volume, along with their roles in treatment decisions.