
Cell One Partners' George Goldberger discusses the issues that new and emerging cell and gene therapy companies face on the road to commercialisation.

Pharm Exec speaks with Geoff MacKay, co-founder and CEO of AVROBIO, about the company’s mission to advance potentially curative lentiviral-based gene therapies, his career building businesses and innovation in regenerative medicine, and his most important quest to date-moving gene therapy into the mainstream.

Cell One Partners' George Goldberger discusses the issues that new and emerging cell and gene therapy companies face on the road to commercialisation.
Jan Lichtenberg, Ph.D, and Scott Friedman, MD, talk about the growing interest in nonalcoholic steatohepatitis (NASH) in the pharma industry, the complexities of modeling the disease, and what this means for drug development efforts.

Getting patient insights before launch is more feasible than you might think.

With little momentum in developing new new antibiotics to fight antimicrobial resistance, the time is now to emphasize "push" and "pull" incentives and demonstrate how innovation in this area will lead to patient benefit and economic reward.

Sabina Heinz and Elizabeth Baynton look at how the continued absence of approved products affecting the management of NASH patients.

Repurposing existing technology to alleviate traditional ‘pain points’ in ensuring clinical investigator payments transparency

Jianan Huang discusses how the drug revenue formula is being used to guide R&D "rescue strategies".

Private equity investment and venture capital have long spurred R&D efforts for hard-to-treat conditions. Joining the mix of late has been impact investing and the opportunities for the socially-conscious to influence drug development-and reap potential benefits in health outcomes and financial return.

Given the complexities of clinical trials, however, accurate updating of actual and forecasted site payments for real-time reporting remains one of the largest challenges in medical product development, writes Shaun Williams.
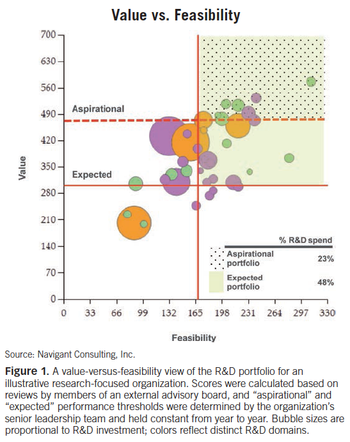
Weighing the benefits of a deeper quantitative review for pharma.

Clinical trials for precision therapies will require a healthy dose of education, collaboration, and data-driven decision-making from everyone involved in the process―not to mention a dedication to digital technology. Justin Grossman reports.

The importance of patient advocacy in boosting research for cystic fibrosis.

Sonalee Agarwal, head of value and evidence strategy for Alnylam, talks with Pharm Exec about the role of real-world evidence in the rare and ultra-rare disease space.
As CAR-T therapy eyes new territory-solid tumors-and expands into autoimmune disease, other frontiers in drug development are beginning to open up. They highlight the raw promise of science, with cannabis-based agents targeting CNS and rare genetic disorders, as well as the larger responsibility to public health, including advancing non-opioid alternatives, addressing antibiotic, and untangling the path to market for biosimilars.

Amid a still-difficult environment for enforcing cannabis-related patents, this article explores some of the types of patent protection available for cannabis-based therapies and inventions.

As this year's Pipeline Report illustrates, there remains an imbalance between funding and incentivizing new drug discovery in some therapeutic areas and rewarding the risk-based nature of these innovations. Imagine if we could solve this conundrum in science?

Pharm Exec examines the booming life sciences scene in southeast Virginia and Raleigh-Durham and Winston-Salem, North Carolina-and the region’s advantages in location, talent, and funding compared to the more glamour spots up north.
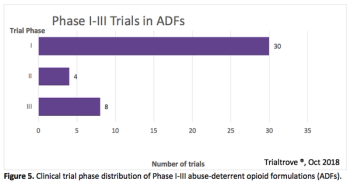
Analysts from Informa Intelligence take a deeper dive into these three pivotal areas of R&D today-the crisis points and progress.

Data shows cultural gaps and highlights a C-level fix.




As this year's ESMO gets under way, Pharm Exec spoke to James Campbell-global franchise business head at Merck KGaA, Darmstadt, Germany-to discuss his vision for the company as a global leader in oncology.

Small biotech companies benefit more from partnering with CROs rather than hiring a chief medical officer.

How one nonprofit and its out-front leader are joining forces with industry to advance a new personalized medicine approach for cystic fibrosis patients nonsense mutations.