
William Looney reviews EvaluatePharma's World Preview 2015 - Outlook to 2020, launched at last week's BIO International Convention.

William Looney reviews EvaluatePharma's World Preview 2015 - Outlook to 2020, launched at last week's BIO International Convention.
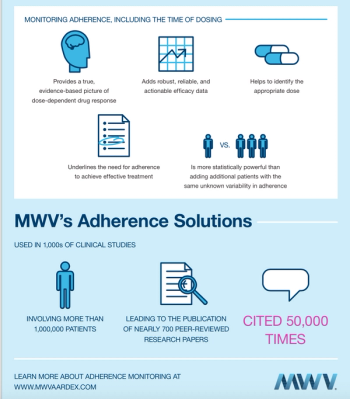
Drug development has never before been so difficult, time consuming and expensive. Accuracy in clinical trials, therefore, is a priority

Masters of the deal converge in New York to discuss the status of their trade and the forces propelling M&A, licensing, and partnerships in the life sciences for 2015.

Susan Crowley reviews the hotly debated agenda items from last month's World Health Assembly (WHA) in Geneva, Switzerland.


This article originally appeared on Applied Clinical Trials.

Emerging technology partnerships in the biopharma space may be provide hope in accelerating patient enrollment, writes Michael Christel.

The concept of increasing the speed and efficiency of clinical trials is a well understood and agreed upon priority of most clinical operations professionals.

Current clinical trials are regarded as “too slow, too expensive, not reliable, and not designed to answer the important questions,” according to FDA’s new deputy commissioner for medical products &tobacco, Robert Califf.

Why executives should make the US R&D tax credit part of their planning discussions, by Chai Hoang and Chris Bard.
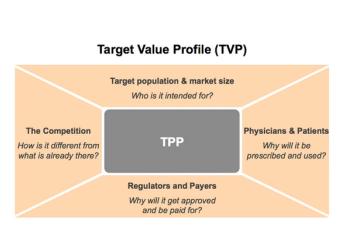
Meike Wenzel and Clifford Hall examine a new concept for early commercialization planning to facilitate good cross-functional working practice.

GlaxoSmithKline announces global vaccines research and design facility to be based in Rockville, MD, USA.

Moe Alsumidaie looks at data collection methods and concepts that can result in predicting patients at risk of dropping out from a clinical trial.

The US drug regulatory system fails to address the country’s most urgent medical needs with the resources appropriate for the task. But change is possible.

According to experts on Pharm Exec's Editorial Advisory Board, 2015 is set to be another interesting year from a consolidation perspective.

Juan Pablo Bagó, general director of pharma in Argentina for the Bagó group, discusses the state of R&D in Argentina, and explains why his group is planning for a 75 percent of turnover from international markets in the coming years.

Amid a resurgence in drug development for hard-to-treat conditions, the bigger question is whether the times are as good for the industry tasked with rendering basic science into therapeutically effective medicines.
New research on the role of microbes in fighting disease is transforming the way medicine views bacteria, writes Lee Jones.

Pharma backs federal standards for compassionate use, drug importing, data transparency, and track-and-trace. Jill Wechsler reports.

Its only recently that vaccine producers experienced the commercial returns commensurate with vaccines: long record of positive public health performance.

The potential of comparative effective research in transforming healthcare remains untapped, but the opportunities are there, writes Michael Christel.

The Cancer Research Institute is clearing a path to the future of immunotherapeutics. Ben Comer speaks to its CEO and director of scientific affairs, Jill O'Donnell-Tormey

Elderly patients are underrepresented in clinical trials, but there are few obstacles to including this patient group in trials that cannot be overcome, writes Sydney Rubin.

In light of the risks and challenges inherent in the development of oncologics, some pharmacos are questioning whether it remains an attractive therapeutic area in which to invest its resources.

Four commercial line executives serve as our jury of peers on what's in store for the future of pharma, and discuss the changing criteria for market success, from drugs to consumer products to vaccines.