
Highlights from Peter Young's conversation with Dr. Steven Miller, Chief Medical Officer and SVP of Express Scripts, on Miller's business philosophy and the current issues facing the sector.

Highlights from Peter Young's conversation with Dr. Steven Miller, Chief Medical Officer and SVP of Express Scripts, on Miller's business philosophy and the current issues facing the sector.

The influx of treatment breakthroughs and expanding science has not silenced the debate over the productivity of biopharma's R&D model, and the looming challenges for R&D decision-makers.

Michael Gordon charts the evolution of regulatory operations and suggests how it can spearhead opportunities for innovation in pharma data.

When lobbying tips the scales: a path to drug approval.

Pharm Exec convenes an expert Roundtable to discuss one of the hottest topics in big data today-how to make that data relevant to payers, regulators and the patient through reliance on real-world evidence in driving better health outcomes.

Pharm Exec sits down with Anthony Marucci, co-founder and CEO of Celldex, a stealth player in the immunotherapy field.

Pharm Exec and inVentiv Health convened a roundtable discussion at the 2016 ASCO meeting to review the use of patient-reported outcomes (PRO) in the clinical trial and commercialization space.

A significant change is under way as the industry shifts from passive to active trial master file (TMF) management, writes Rik van Mol.

Dave Handelsman offers a smart approach to dealing with the EMA's new guidelines on the anonymization of clinical trial data.

Good Distribution Practice means that ‘track and trace’ is becoming indispensable for drug product integrity. Interactive Response Technologies may offer a potential solution.
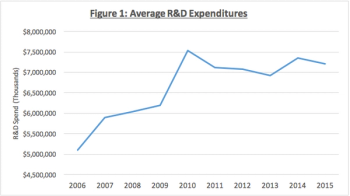
Amidst arguments that industry is heading for another economic recession, Moe Alsumidaie reminds us that biopharma has shown resiliency during such times.

As new ICH GCP draft guidelines now require root cause analysis, novel methods for risk analysis and triage must be adopted in drug development.

With the emerging industry commitment to publicly disclose research and clinical trial results, two pivotal issues come to mind - and serve as a reminder to companies that true transparency depends on the truthfulness of the evidence that binds it.

A new wave of technologies supported by innovative business models is transforming the vaccine landscape - and raising the bar on performance. As the demand for cures for chronic diseases accelerates, and with more global outbreaks of viral diseases like Zika and Ebola a virtual certainty, solutions can’t come soon enough.

Can novel drug delivery technologies offering options beyond the traditional tablet or syringe transform the therapeutic experience for patients?

Vaccine R&D has grown exponentially in recent years, spurred by ethical and medical needs to combat lethal infectious outbreaks and increased funding from public and private agencies and organizations.

Canada’s Rx&D, the industry’s oldest functioning trade association, recently changed its name-with a mission of bridging the many points of collaboration that drive the process of modern drugs, from discovery to market.

A closer reading of this year's JP Morgan investor conference identifies three areas where the insular, often maladroit tone of the industry-investor dialogue may be morphing to something more grounded in the larger societal context of healthcare.

Drug pricing backlash threatens regulatory reform, R&D advances



For years, many companies have competed to demonstrate that their products could achieve the holy grail in type 2 diabetes (T2D): cardiovascular (CV) risk reduction. According to the American Diabetes Association, pharma companies to date have spent over $2 billion and tested 138,000 patients in company-sponsored CV-risk reduction T2D trials, including the recent large-scale TECOS, SAVOR, EXAMINE, and ELIXA studies.

The Alliance for Clinical Research Excellence and Safety (ACRES) is advocating a “systems thinking” approach in efforts to meet increasing demands to standardize and integrate support for trial sites.

Casey McDonald previews pharma’s Oscars, the Prix Galien USA Awards, which this fall will bring some red carpet glamor and, hopefully, cheer to the industry.

September 28, 2015.