
A common takeaway in our exploration of "Emerging Biopharma" appears to be that these companies are those that go beyond pure discovery-and put equal efforts into the pivot to potential commercialization.

A common takeaway in our exploration of "Emerging Biopharma" appears to be that these companies are those that go beyond pure discovery-and put equal efforts into the pivot to potential commercialization.

While the field of regenerative medicine has grown considerably since the ’90s-in innovation and acceptance-entrenched companies such as Athersys are looking to forge new advances in the stem cell arena by transforming promising science into real-world treatments.

Streamlined clinical research, more guidance speed new cures to patients.
In its 12th annual feature, Pharm Exec builds on the revamped approach it introduced last year in spotlighting notable biopharma brands, profiling a new round of products that are making waves in five key areas in healthcare and R&D.

Amid new efforts to inspire more physicians to engage their patients about clinical research, turning that vision into tangible gains in trial participation remains tricky.
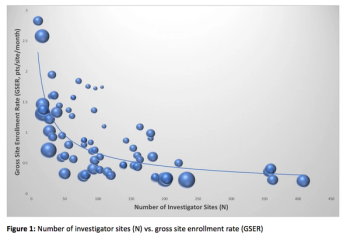
A discussion of the cost and time put into clinical trials.

The biotech sector has managed to elude the negative perceptions saddling traditional pharma these days. We examine the reasons why.

Improving the productivity of sponsors’ clinical development processes will continue to be a primary driver of outsourcing in 2018, writes Nuala Murphy.

The advent of immunocellular therapies marks a major milestone in pharma, but the path for these treatments in transforming patient care is still forming as they face challenges in clinical trials, pricing, and market acceptance,

Sean McCarthy, President & CEO of CytomX, outlines the company’s journey from university startup to rising star of the immuno-oncology space.

New action plan urges tighter alignment of public health policy with the development of targeted therapies.

Analysis shows that persistence is paying off for drug developers, driven by the rise of CAR-T and other gene therapy, newly discovered cancer targets, better patient identification methods-and the realization that failures have their place in shaping the pipeline of tomorrow.

is spectacular science enough to guarantee success for new medicines?
The Ipsos Healthcare Oncology Centre of Excellence's Jackie Ilacqua & Valerie Wriede trace the evolution of cancer treatment and drill down into the latest developments currently redefining the landscape, together with those on the horizon.

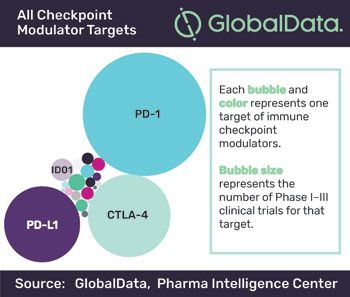

Even on the contentious topic of orphan drug pricing, the last few months have brought hopeful signs that patients, pharmaceutical companies, health insurers, and the academic bodies that counsel them are trying to speak the same language.


Dogs and kids both spontaneously develop a number of cancers, making it a no-brainer for those on both sides of the disease to work together in finding new therapies.




With the patient voice growing louder in all aspects of the drug development and commercialization journey, Pharm Exec examines some of the current industry thinking on the evolving pharma-patient relationship.

