The novel therapy, ABD-147, uses advanced antibody engineering to deliver Actinium-225 to solid tumors expressing DLL3, a protein found on neuroendocrine tumors.

The novel therapy, ABD-147, uses advanced antibody engineering to deliver Actinium-225 to solid tumors expressing DLL3, a protein found on neuroendocrine tumors.
DSP-5336 targets the menin and mixed-lineage leukemia protein interaction, crucial in various biological processes, including cell growth and genomic stability.
Clearance of the New Drug Application for ART25.12 allows Artelo to begin a Phase I study for the drug in chemotherapy-induced peripheral neuropathy.

According to the Complete Response Letter, the FDA has requested additional information on the manufacturing process and the type 1 diabetes indication for insulin icodec before completing its review.
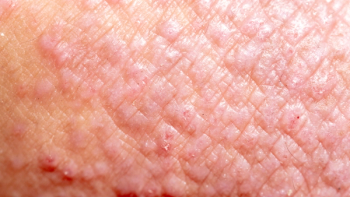
Zoryve is a steroid-free, once-daily treatment shown to offer rapid disease clearance and significant itch reduction for patients with mild to moderate atopic dermatitis.
Approval of the Mynx Control Venous vascular closure device was based on results from the ReliaSeal trial, which demonstrated 100% procedural and device success in cardiac ablation procedures.
Results from the Phase III STREAM Stage 2 study show the efficacy and safety of an all-oral bedaquiline-containing regimen for multidrug-resistant pulmonary tuberculosis.

The Vabysmo prefilled syringe is designed to simplify administration for retina specialists, improving the treatment experience for both physicians and patients with wet age-related macular degeneration, diabetic macular edema, and macular edema following retinal vein occlusion.

Kisunla is the first amyloid plaque-targeting therapy that allows for stopping treatment upon plaque removal, company says.
The Fast Track designation for INZ-701 was based nonclinical pharmacology data and preliminary safety and efficacy data from the ongoing Phase I/II trial of INZ-701 in adults with ABCC6 Deficiency.
Reportedly, the liquid formulation of Tepylute eliminates the need for complex and time-consuming reconstitution, providing consistent dosing accuracy and allowing for timely preparation.

Approval of both NRX-101 and NRX-100 could potentially yield more than $150 in revenue per NRXP share in the near term, company says.
The inclusion of Vaxelis in the CDC’s preferential recommendations is expected to influence vaccine administration strategies and public health policies in high-risk populations moving forward.
Ohtuvayre is the first inhaled product with a novel mechanism of action for chronic obstructive pulmonary disease to be approved in 20 years.
In an interview with Pharm Exec Associate Editor Don Tracy, Leonard Mazur, Co-founder, CEO, Citius Pharmaceuticals, offers an update on the recently accepted Biologics License Application for Lymphir by the FDA and other candidates currently in the Citius pipeline.
The Complete Response Letter was issued as a result of inspection findings at a third-party manufacturing facility, unrelated to patritumab deruxtecan’s efficacy or safety in patients with advanced or metastatic EGFR-mutated non-small cell lung cancer.
Approval of Epkinly was based on results from the Phase I/II EPCORE trial in patients with relapsed or refractory follicular lymphoma who have already completed two or more lines of systemic therapy.

Results from three randomized clinical trials show safety and efficacy of a brexpiprazole-sertraline combination in adult patients with post-traumatic stress disorder.

Cordella is the first pulmonary artery pressure-guided platform indicated for home-based comprehensive heart failure patient management.

Approval of Wakix marks the first time a non-scheduled treatment option for excessive daytime sleepiness has been approved for patients ages 6 years and older.
Accelerated approval for Krazati is based on results from the Phase 1/2 KRYSTAL-1 study, which demonstrated a 34% objective response rate in patients with previously treated, locally advanced, or metastatic colorectal cancer.
Expansion of Elevidys includes patients over the age of four years with Duchenne muscular dystrophy regardless of their ambulatory status.

Approval of Sofdra marks the first chemical entity to be approved by the FDA to treat primary axillary hyperhidrosis.

Approval of Skyrizi marks the first IL-23 specific inhibitor approved for both ulcerative colitis and Crohn disease of similar severity, according to AbbVie.
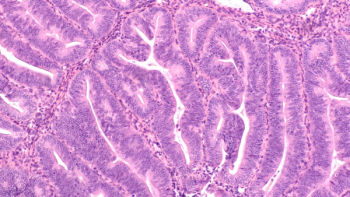
Approval of Keytruda combination for patients with primary advanced or recurrent endometrial carcinoma is based on results from the NRG-GY018/KEYNOTE-868 clinical trial, which was Phase III trial to evaluate an anti-PD-1 immunotherapy plus chemotherapy combination in patients with two distinct tumor types.