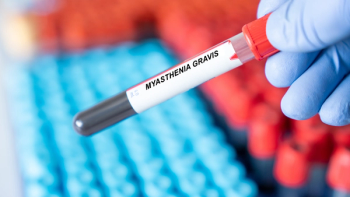
Priority Review was based on results from the Phase III Vivacity-MG3 study of nipocalimab in patients with generalized myasthenia gravis.

Priority Review was based on results from the Phase III Vivacity-MG3 study of nipocalimab in patients with generalized myasthenia gravis.
Abrysvo and Arexvy will now be required to come with labeling that includes a warning about a potential increased risk of Guillain-Barré Syndrome.
Fast Track designation was based on results from a Phase II study, which demonstrated that VGT-309 was safe, well-tolerated, and enhanced tumor visualization in lung cancer patients.
Breakthrough Therapy Designation for GSK5764227 was based on data from the ARTEMIS-002 Phase II trial, which demonstrated promising efficacy and safety in 42 osteosarcoma patients.
The designation marks VGA039 as the first drug candidate to receive FDA Fast Track designation for von Willebrand disease.
Approval of Tevimbra was supported by the RATIONALE-305 Phase III trial, which demonstrated a significant overall survival benefit in patients with unresectable or metastatic HER2-negative gastric or gastroesophageal junction adenocarcinoma.
Approval was based on results from the Phase III CheckMate-67T trial, which demonstrated non-inferior pharmacokinetics compared to intravenous Opvido.

In this part of his Pharmaceutical Executive video interview, Peter Ax, CEO of UpScriptHealth, discusses regulatory challenges and opportunities that telehealth companies face in 2025.

Approval marks the first generic version of liraglutide injection, referencing Victoza, for improving glycemic control in adults and children aged 10 years and older with type 2 diabetes, alongside diet and exercise.
Approval was based on positive results from the Phase III balance study, where Tryngolza demonstrated a notable placebo-adjusted triglyceride reduction at 12 months.
Accelerated approval was based on promising results from the ongoing Phase III BREAKWATER trial.

In this part of his Pharmaceutical Executive video interview, Peter Ax, CEO of UpScriptHealth, discusses the recent DEA extensions of telehealth for controlled substances and new treatment modalities or patient populations he anticipates will become more accessible through telehealth in 2025.
Breakthrough Therapy designation for Trodelvy was granted based on promising results from the Phase II TROPiCS-03 study, which showed encouraging antitumor activity in both platinum-resistant and platinum-sensitive extensive-stage small cell lung cancer.
Approval of Steqeyma was based on a comprehensive evidence review, including a Phase III trial in moderate to severe plaque psoriasis.
New biologics license applications seek FDA approval for Merck’s clesrovimab to protect infants and children from respiratory syncytial virus and Johnson & Johnson’s Simponi for the treatment of ulcerative colitis.
Approval of Vtama for atopic dermatitis provides a steroid-free treatment option for adults and pediatric patients.
The Breakthrough Therapy Designation for Jemperli was based on Phase II clinical trial data, showing a 100% clinical complete response rate in patients with locally advanced mismatch repair deficient/microsatellite instability-high rectal cancer.
With the Inflation Reduction Act now in flux, how will the government and industry respond?
Approval of Unloxcyt was based on results from the CK-301-101 trial, which showed the drug is the first FDA-approved PD-L1–blocking antibody to produce clinically meaningful and durable objective response rates in patients with metastatic or locally advanced cutaneous squamous cell carcinoma.

The importance of expanding your pharma sales forecasting horizon beyond the US. Learn how to effectively navigate country-specific regulatory red flags.

The regulatory action was supported by results from the Phase III HERCULES trial, which demonstrated a 31% reduction in six-month confirmed disability progression in non-relapsing secondary progressive multiple sclerosis.
Real-world evidence from the largest analysis of second-line treatment with Yescarta in 2022-2023 demonstrated a high overall survival rate in patients with relapsed/refractory large b-cell lymphoma.
Breakthrough designation was based on results from the TROPION-Lung05 Phase II trial with support from the TROPION-Lung01 Phase III trial of datopotamab deruxtecan in patients with locally advanced or metastatic epidermal growth factor receptor-mutated non-small cell lung cancer.
Ziihera’s addition as a category 2A treatment follows the FDA’s accelerated approval of the drug for adults with previously treated, unresectable, or metastatic HER2-positive biliary tract cancer.
The FDA based the Priority Review designation on results from the Phase III NIAGARA trial, which found that Imfinzi reduced the risk of disease progression, recurrence, or death by 32% in patients with muscle-invasive bladder cancer.