
The cobas is classified as a four-in-one molecular test that can identify SARS-CoV-2, influenza A, influenza B, and respiratory syncytial virus from a single nasopharyngeal or anterior nasal swab sample.

The cobas is classified as a four-in-one molecular test that can identify SARS-CoV-2, influenza A, influenza B, and respiratory syncytial virus from a single nasopharyngeal or anterior nasal swab sample.

Approval for Kevzara was based on controlled studies, pharmacokinetic data from adults with rheumatoid arthritis, and pediatric-specific studies on pharmacokinetics.
Klisyri is now available in a 350 mg package size as a topical treatment for actinic keratosis on the face or scalp.

Arexvy receives expanded indication to include adults aged 59 years and younger to prevent RSV lower respiratory tract disease.

Approval of Rytelo could provide patients with lower-risk myelodysplastic syndromes with extended periods without the need for red blood cell transfusions to alleviate symptomatic anemia.
According to the manufacturer, the LFR-260 features advanced technology that allows patients to compare multiple prescriptions simultaneously, eliminating the need for traditional refraction methods.

A similar application was also submitted to the EMA for the expanded use of Prezcobix to treat children aged 6 years and older with HIV.

In a majority vote, the Psychopharmacologic Drugs Advisory Committee cited concerns regarding the data presented for MDMA as a treatment for post-traumatic stress disorder.

EVERSANA INTOUCH Media’s EVP discusses how the ban may impact the industry and how companies can prepare for it.

Approval of Onyda XR marks the first liquid non-stimulant medication for attention-deficit hyperactivity disorder to hit the market in the United States.
Breyanzi, a CD19-directed CAR T-cell therapy, granted fourth FDA approval to treat a distinct subtype of non-Hodgkin lymphoma.
Submission for the FDA approval of zanidatamab was based on promising data from the Phase IIb HERIZON-BTC-01 clinical trial in patients with previously treated, unresectable, locally advanced, or metastatic HER2-positive biliary tract cancer.
Priority review designation for Keytruda is based on promising results from the Phase II/III IND.227/KEYNOTE-483 trial, which demonstrated improved overall survival in patients with unresectable advanced or metastatic malignant pleural mesothelioma.
Priority Review status for inavolisib is based on positive Phase III data showing the inavolisib-based regimen significantly extended progression-free survival in patients with hormone receptor-positive, HER2-negative breast cancer with a PIK3CA mutation.
Fast track designation for AV-001 comes amid promising results from a Phase I trial, which supported once-daily dosing and effective Tie2 activation.
Moving forward, Durect plans on incorporating feedback from the FDA in the design of a Phase III trial.

Approval of Yesafili represents Biocon’s entrance into the US ophthalmology market, following previous approvals in Europe and the UK.
Imdelltra (tarlatamab-dlle) is the first T-cell engager therapy approved for extensive stage small cell lung cancer.

This is the second accelerated approval the FDA has granted to Bristol Myers Squibb’s chimeric antigen receptor T-cell therapy in the past few months.

Carta Healthcare’s co-founder discusses how a recent ruling on non-compete clauses may help alleviate the coming nursing shortage.
Roche’s human papillomavirus solution is reportedly one of the first of its kind to be available in the United States.
Dupixent has previously been approved for adults with chronic rhinosinusitis with nasal polyposis whose condition is inadequately controlled.

With competition for follow-on-biologics on the upswing—and a potential market boom perhaps around the corner—continued education and course-setting for all stakeholders in charting the access landscape is paramount.
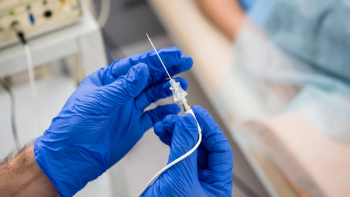
The catheters approved by the FDA include the Plato 17, a DMSO-compatible microcatheter, and the Socrates 38, an aspiration catheter specifically designed for treating ischemic strokes.

The founder and CEO of UpScriptHealth discusses the issues causing the shortage and how recent decisions from the DEA may help solve the issue.