
To win consumers' trust and loyalty, pharma companies should look to the privacy regulations of the Health Insurance Portability and Accountability Act of 1996 (HIPAA) to guide their direct-to-consumer (DTC) and web strategies.

To win consumers' trust and loyalty, pharma companies should look to the privacy regulations of the Health Insurance Portability and Accountability Act of 1996 (HIPAA) to guide their direct-to-consumer (DTC) and web strategies.

In a closely watched patent case that has important implications for pharma companies, the Supreme Court ruled unanimously in May to uphold policies that protect patent holders from imitators. The decision, in what is considered one of the most significant patent disputes to come before the court, is expected to benefit brand-name companies that bring patent infringement cases against generics makers.

As part of an aggressive campaign against the rising cost of medicines, AARP, the national advocacy group for Americans over 50, joined three class-action lawsuits against pharma companies involving alleged anticompetitive efforts to block generic competition and inflate prices.

Setbacks sometimes prompt a great leap forward. For Sankyo, a major product withdrawal-most painfully, of its potential blockbuster for diabetes, Rezulin (troglitazone)-created the reversal. Major liver effects appeared in the market

This season, boosterism is out; hedging is in. Yet a realistic assessment of the pharma market, even under the shadow of terror and war, reveals new opportunities. For every ominous sign or setback, a potential line of offense exists. Despite the press of a slow economy, nervous investors, customer consolidation, and cost-shifting in healthcare, the industry remains rich in resources, growth, and opportunity. Here are some of the market forces, issues, and opportunities for the pharma universe this year:

With the huge costs of developing a new drug from scratch and the high attrition rates as lead compounds fail during the trials process, in-licensing has become an increasingly popular method for pharma companies to boost their pipelines without all of the outlay involved in de novo research.
FDA has issued the industry a new charge-pay closer attention to risk management. Now that prescription drug user fees have helped the agency approve candidates more rapidly, FDA has returned to its basic mandate: assuring that marketed pharmaceuticals are safe. In the past, that meant clear labeling with adequate directions and warnings based on clinical trials. The agency now believes that product safety extends beyond warning labels and wants to ensure that prescriptions are used safely as well. As a result, it is asking the pharma industry to demonstrate products' safety before approval and to further control their use after

A former Warner-Lambert employee has blown the whistle on the company's "shadowing program," alleging that some physicians accepted money in exchange for allowing pharma sales representatives to meet with patients, review charts, and recommend prescriptions. According to the lawsuit, Warner-Lambert-since acquired by Pfizer-tried to boost sales of its epilepsy drug Neurontin (gabapentin) by tracking prescriptions and rewarding high-prescribing physicians with gifts such as cash, trips to resorts, and lucrative speaking and consulting jobs-as well as paying them to enter patients in clinical trials. The program allegedly paid 75-100 US doctors at least $350 per day to let sales reps watch

The decoding of the human genome and its potential to open the door to cures for AIDS, cancer, and many other conditions that today are incurable are likely to shapethe healthcare industry for decades to come.

In today's legal climate, pharmaceutical companies are prime targets for litigation in both state and federal courts. Because they are engaged in product research and development and related complex business practices spread across many jurisdictions and because the public perceives the industry as having deep pockets, executives must be prepared for-and frankly must expect to face-the challenges of major multijurisdictional litigation.
Does FDA harm patients by rushing unsafe products to market or by slowing product development and approval? That debate came to the fore recently with the almost simultaneous release of competing studies.

Terminally ill patients often wonder about the roles of timing and fate in determining their life's course. If only they had been tested or diagnosed earlier; if only the doctors had found the tumor before it metastasized. As purveyors of science and administrators of public health, the world's pharma companies and physicians struggle to intervene earlier-indeed to predict and prevent disease-before it's too late.

In April, a distinguished group of scientists and legal experts gathered in San Francisco to discuss two of the most exciting and controversial research topics of the century: stem cell research and xenotransplantation.

During the next three years, an estimated $38 billion in brand-name pharmaceuticals will come off patent, leaving a financial void that many pharma companies hope to fill with functional genomics.

Pricing has never been more of a key issue for the industry than it is right now. Yet, even with the increased importance of pricing strategies, a lack of focus on critical market factors leads many manufacturers to forego profits or increase their vulnerability to aggressive payers. Aligning pricing and contracting can achieve a sustainable competitive advantage-if product managers objectively assess a product's clinical benefits and address two key questions:

The last few years have seen tremendous consolidation in both the pharmaceutical and contract research industries. The impact among pharma companies has created a heightened demand for productivity. Consequently, contract research organizations (CROs) have struggled to find their footing in a business where the number of customers has shrunk and the demand for speed and cost-effectiveness has risen. Delivering service excellence when customers' names and addresses are changing regularly is a challenge, resulting in disrupted continuity, broken lines of communication, and policies and relationships thrown into disarray.

First Amendment protection of commercial speech got a big boost last month when the US Supreme Court ruled 5-4 that FDA's policy to limit advertising for pharmaceutical compounding is unconstitutional.

International trade rules play a large role in creating world poverty, according to Oxfam, an international confederation of organizations committed to end poverty. In a recent report, "Rigged Rules and Double Standards," the group accuses rich nations of robbing poor nations of $100 billion a year by abusing trade rules. It also criticizes pharma for enforcing its patents in poor countries.

The past few years have seen a steady increase in new medicines being discovered and developed in the United States and a decrease in those from Europe, where it takes much longer for new medicines to become available to patients. In some cases, it takes medicines three or four years from their approval date to reach the market in all European Union member states. So, in March 2001, the European Commission appointed a high-level group to address those issues on a Europe-wide basis, involving all the different member states and stakeholders.
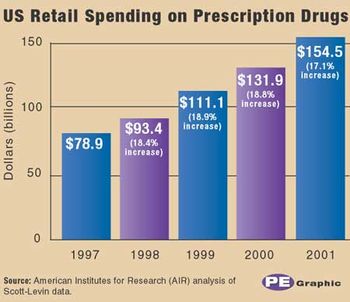
The National Institute of Health Care Management made headlines with its report on double-digit increases (17 percent) in retail spending on medicines in 2001. The total reached $155 billion last year, almost double the $80 billion spent in 1997, according to the study, "Another Year of Escalating Costs." PhRMA president Alan Holmer said the increase is a good thing, signifying that more people who need medicines for chronic conditions are being treated and thereby avoiding more expensive medical procedures.

African-American physicians regard direct-to-consumer advertising of prescription medicines as one way to educate minority patients about needed treatment and healthcare options, according to a survey conducted by the National Medical Association (NMA). Almost all of the 900 physicians answering the questionnaire reported that DTC advertising has prompted patients to ask questions, and one-third acknowledged that they feel additional pressure to justify their prescribing decisions. But almost half (48 percent) said that such promotion increased communications between physicians and patients.

Among the dozens of initiatives in Washington affecting the pharma industry, one common thread is the growing movement to control accelerating expenditures for prescription medicines.

Science deserves all the credit it gets, and more, for driving this industry. Still, no science succeeds without a certain application of what we broadly call art. Inside the scientific discipline, many arts apply-the art of experiment, the art of planning, and the art of free association among them.

The returns are in, and they are not pretty. Janus Funds' billion-dollar investment in WebMD in 2000 now has a net value of roughly ten cents on the dollar, and many observers see WebMD as a winner in their space-whatever that space is now.

The European Medicines Evaluation Agency (EMEA) has promised to get tough with pharma companies if they fail to follow its rules for reporting product side effects. It may resort to naming and shaming those that do not comply and could even prosecute offenders.

Kiln, a Lloyds of London underwriter, has come up with a novel way for pharma companies to mitigate the risk of patent challenges: insurance. Kiln created a policy for a big pharma company covering a number of patents and trademarks the company considers essential for its future profitability.

As the fanfare over the sequencing of the human genome fades, the spotlight turns to the "shovel and pans" companies offering the tools investors are betting on to make "gene to drug" a reality.

Products in Trouble

Washington DC-In addition to launching an ad campaign backing adoption of a Medicare drug benefit, PhRMA is expanding its state lobbying efforts to block local prior authorization requirements. As more states face huge shortfalls in Medicaid budgets because of declining revenues and rising healthcare costs, they seek to cut spending on prescriptions. That means more restricted formularies and rebate requirements, with prior authorization imposed on doctors to limit prescribing of therapies that fail to offer sufficiently attractive deals.

Canberra, Australia-The Australian Pharmaceutical Manufacturers' Association has denounced calls from the country's media and some of its doctors to drastically curtail drug promotion. APMA chief executive Alan Evans says any such move would severely affect the healthcare of millions of people in Australia and could even result in premature death.