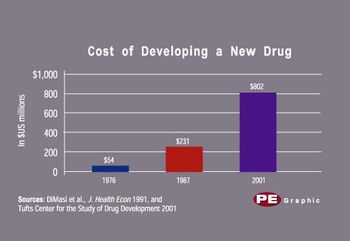
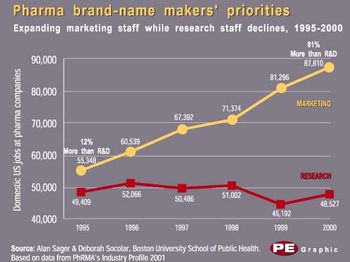
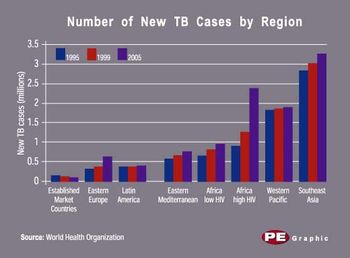
Who, or what, creates wealth? Answering that question has become as much political football as economic theory. Last month, the US president reminded us that government does not make wealth but, at best, fosters a climate conducive to it. The old-left idea that workers create and should share equally in the fruits of production has long since died of exhaustion. By process of elimination, the only apparent answer remaining belongs to idealistic capitalists, who herald the enterprising companies from which all wealth "obviously" flows-for most of us, as paychecks.