
Pharm Exec speaks to ICON's Ramita Tandon about how the UK and European regulators' market access plans for 2017 will affect the industry.

Pharm Exec speaks to ICON's Ramita Tandon about how the UK and European regulators' market access plans for 2017 will affect the industry.

With the push toward value-based heathcare set to accelerate even more in 2017, Takeda’s VP of global market access discusses the evolution of this all-important engine for medical innovation and bottom-line returns.

Pharm Exec speaks with Ramita Tandon, executive vice president, ICON Commercialization & Outcomes, about the potential impact of UK and European regulators’ market access plans for the industry.

Despite serving as a regional hub for numerous leading global healthcare and life sciences companies-while simultaneously straddling the frontier of biomedical advancement and innovation-Singapore is reinventing itself yet again and spearheading a new model of Asian healthcare.

As the healthcare landscape evolves, pharmaceutical companies are being driven to embrace new, unique partnerships to go beyond the pill, writes Weng Si Ho.

The long-cherished dream of finding a European approach to assessing the value of new medicines seems to recede further with every step taken to pursue it, writes Reflector.

The Lancet's series of papers on moving from universal health care coverage to "right care" for health offers a truly global and comprehensive perspective, writes Leela Barham. But just what does "right care for health" mean for the industry?

This sponsored supplement was produced by Focus ReportsProject Publisher: Mariuca GeorgescuProject Director: Carla Verdera MateuCoordinators: Brandon Mourich & Julija LukaityteProject Assistants: Zoë BerginSenior Editor: Louis HaynesEditor: Patrick Burton

The pursuit of a harmonized European approach to assessing the value of new medicines continues to face roadblocks.

2016 has been the year of the unpredictable - and the uncertainties this has generated will multiply and dominate the agenda in Europe in 2017, writes Reflector.

In a continent marked by stark differences in governance, growth and market potential, the best bets must be pursued off script.

How a strong corporate board can help ignite success in these regions.

Leela Barham speaks to NICE International and Imperial College’s Institute of Global Health Innovation about joining forces under the International Decision Support Initiative.

In a regional context marked by chronic instability and turmoil in strategic Latin American economies such as Brazil and Venezuela, Mexico has continually strengthened its positioning as a destination of choice for the global pharmaceutical industry.

The European Medicine Association is determined keep open all options on the future of drug approvals in advance of a crucial meeting in December, writes Peter O'Donnell.

The UK's NICE, like the NHS it supports, is looking at how to make big savings. One option is to get more "commercial". Leela Barham takes a look.

Leela Barham looks at the 18 recommendations of England’s Accelerated Access Review (AAR), which proposes how to speed up adoption of the best innovation in the NHS.
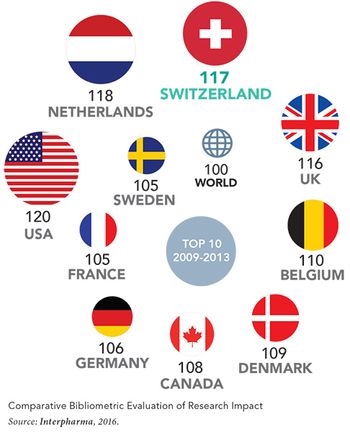
Switzerland’s formidable pedigree as a pharmaceuticals powerhouse and hotbed of scientific innovation is often overlooked. But indeed these elements serve as major linchpins of the Swiss national economy, as the country continues to punch above its weight on the global stage for health affairs and medical-science advancement.

Cancer treatment is an increasing dilemma for health services as they try to balance patient care against budgetary constraints.

The latest price rules revision is both radical and rare by the government’s usual exhaustive standards. What do the new rules mean for pharma players in the world’s third-largest market?

The potential collapse of the CETA agreement jeopardizes EU’s overall trade footing.

The UK's vote to leave the EU was the hot topic - and a source of exasperation - at last week's annual European Health Forum Gastein.

Though comparatively smaller when positioned against some of the country’s neighbors, the pharmaceutical market in the Czech Republic finds itself on a solid growth trajectory, with innovator drug developers grabbing a larger piece of the value pie in the traditionally generics- dominated setting.

The UK is moving closer to aligning its two approaches to regulating drug pricing - the voluntary PPRS and statutory price cuts - but the headaches are likely to continue, writes Leela Barham.

Leela Barham looks at the potential cost to industry of the National Institute for Health and Care Excellence's plans to charge for TAs.