
Pharmaceutical Executive
In general, it will take two or three trials in a new location to develop a solid base offshore.

Pharmaceutical Executive
In general, it will take two or three trials in a new location to develop a solid base offshore.

Pharmaceutical Executive
By leveraging EDC's features, sponsors can manage study progress better, adapt to opportunities, reduce trial times, and monitor patient safety.
Pharmaceutical Executive
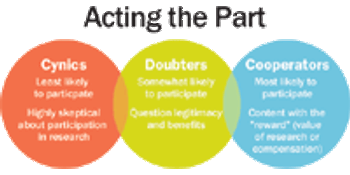
Three friends are talking over lunch. The first person declares, "I'm donating blood," to which his friends reply, "That's good." The second says, "I'm donating an organ." They all agree that it's great. When the third person announces, "I'm participating in a clinical drug trial," his statement is met with silence, confusion, then skepticism.

Pharmaceutical Executive
While execution quality doesn't deal with the protocol from a scientific standpoint, it is nonetheless critical to a successful outcome.

Pharmaceutical Executive
The plaintiffs argued that Amgen breached its fiduciary duty to ameliorate their pain and treat their illness with the best medicine available.

Pharmaceutical Executive
Did you know that the first clinical trial was carried out between 605 and 562 B.C.? According to HealthandAge.com, King Nebuchadnezzar II ordered his subjects to follow a strict diet of meat and wine for three years. It wasn't until four children in his court convinced him to allow them to switch to bread and water instead that an endpoint was reached. After ten days, those in the bread-and-water group appeared resplendent-better nourished than those who had followed the meat and wine regimen.