Bloomlife’s MFM-Pro is a prescription-based wearable device that can track the maternal and fetal heart rate.
Novartis' Lutathera Shows Significant Survival Benefit as First-Line Treatment for GEP-NETs

Bloomlife’s MFM-Pro is a prescription-based wearable device that can track the maternal and fetal heart rate.

Fran Gregory, VP of Emerging Therapies, Cardinal Health discusses her career, how both CAR-T therapies and personalization have been gaining momentum and what kind of progress we expect to see from them, some of the biggest hurdles facing their section of the industry, the importance of patient advocacy and so much more.

HyQvia is the only subcutaneous immune globulin infusion that can be administered once per month.
Vertex Pharmaceuticals' and CRISPR Therapeutics’ Cas9 therapy Casgevy approved as a one-time treatment for transfusion-dependent beta thalassemia on the heels of its approval last month for sickle cell disease.

The data was collected during the LEAP clinical program, developed in partnership with Merck.

Company displayed findings at the 2024 Winter Clinical Dermatology Conference in Hawaii demonstrating significant improvements in atopic dermatitis and seborrheic dermatitis.
Dupixent is an IL-4 receptor alpha antagonist that has been approved for the treatment of adults with moderate-to-severe atopic dermatitis, as well as asthma, chronic rhinosinusitis with nasal polyposis, eosinophilic esophagitis, and prurigo nodularis.

The FDA issued the 39th overall approval for Keytruda (pembrolizumab), with the latest in combination with chemoradiation for the treatment of patients with FIGO 2014 stage III to IVA cervical cancer.
RC88 is under evaluation for the treatment of patients with platinum-resistant recurrent epithelial ovarian, fallopian tube, and primary peritoneal cancer.
Axon Therapy uses magnetic peripheral nerve stimulation to deliver a non-invasive treatment for chronic pain related to nerve damage caused by diabetes.
If the FDA approves the abbreviated new drug application for Lutetium Lu 177 Dotatate, the drug will be eligible for 180 days of generic marketing exclusivity in the United States for the treatment of somatostatin receptor–positive gastroenteropancreatic neuroendocrine tumors.
SELLAS Life Sciences Group, Inc’s CDK9 inhibitor SLS009 is being evaluated in an ongoing Phase I/II study in combination with Venclexta and Vidaza for patients with relapsed or refractory acute myeloid leukemia.

SH-105 eliminates the need for powder reconstitution, which Shorla stated will bolster the novel product’s efficiency and lower the risks associated with drug preparation.
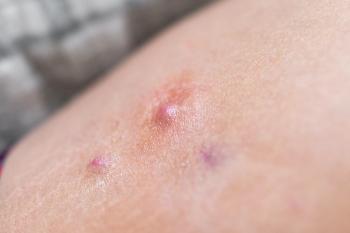
Lutikizumab (ABT-981) will advance to a Phase III trial in patients with moderate to severe hidradenitis suppurativa who previously failed anti-tumor necrosis factor therapy.

Supplemental Biologics License Application would convert the accelerated approval granted to Tivdak (tisotumab vedotin-tftv) to a full approval for patients with recurrent or metastatic cervical cancer with disease progression on or after first-line therapy.
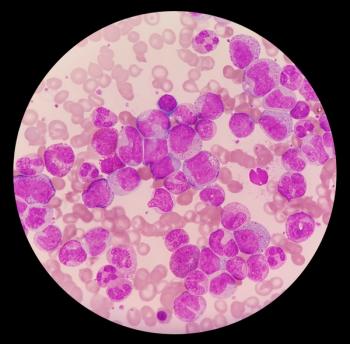
Scemblix (asciminib) plus investigators’ choice of tyrosine kinase inhibitor showed clinically meaningful and statistically significant data in patients newly diagnosed with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase.

Merck is actively enrolling patients across Phase III trials for four novel candidates for hematologic malignancies and solid tumors.

Cretostimogene grenadenorepvec (CG0070) is currently being evaluated for the treatment of patients with high-risk Bacillus Calmette-Guérin–unresponsive non–muscle invasive bladder cancer with carcinoma in situ with or without Ta or T1 tumors.
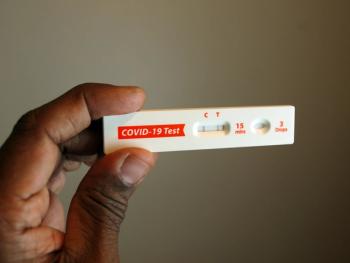
VYD222 is a broadly neutralizing, half-life extended monoclonal antibody developed specifically to prevent COVID-19 in immunocompromised adults and adolescents.
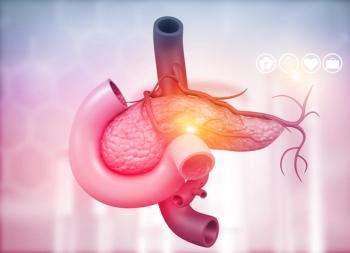
Study results show an estimated 71.4% survival rate after both 24 and 36 months with aglatimagene besadenovec (CAN-2409) in combination with valacyclovir for the treatment of patients with pancreatic ductal adenocarcinoma compared with 16.7% in the control group.

If approved by the FDA, Xolair would be the first drug indicated to lower allergic reactions to multiple foods after an accidental exposure, including peanut, milk, and egg allergies.

Filsuvez (birch triterpenes) is indicated to treat partial thickness wounds associated with junctional epidermolysis bullosa and dystrophic epidermolysis bullosa.

Garadacimab is a novel, first-in-class, recombinant monoclonal antibody for hereditary angioedema that targets activated Factor XII.
Merck’s Biologics License Application for V116, a novel 21-valent pneumococcal conjugate vaccine has been given a Prescription Drug User Fee Act (PDUFA) date of June 17, 2024.

Indication of Adbry (tralokinumab-ldrm) expanded to include patients 12 to 17 years of age with moderate-to-severe atopic dermatitis whose disease is not adequately controlled with topical prescription therapies or for whom those therapies are not advisable.