The payoff is superior performance in key areas such as clinical trials

The payoff is superior performance in key areas such as clinical trials

42 of the best new drugs in development–or parked at the FDA

Tracking, monitoring, and trend evaluation is not enough. Companies must now do more than assess what happened and why.

Innovating in R&D may not be such a complicated task. Consumers simply want to find the product that gets a job done right every time

A new leader, a big acquisition, and a bold investment in HIV may all be in the works at Gilead. But what will it take to restore the glory days?
Industry needs to engage in a broader public debate on ways to rekindle the innovative engine in new drug discovery and development

Pfizer's VP and Assistant General Counsel for Global Patents and Policy, Roy Waldron, discusses its collaborative strategy to refresh the face of IP

Professor Bill Trombetta takes a snapshot of the "Glamour 24" companies shaping the pharma industry this year and beyond

The European Generic Medicines Association's Director for Scientific Affairs, Suzette Cox, talks to us about the environment for biosimilars.

May 24, 2010.

Re-examining service provider roles can bring safety and speed on the road to registration.

Several leading pharmas may already have a drug for a retrovirus linked to chronic fatigue disease. Has medicine's "problem child" finally earned the industry's respect?

How proper site activation and site enrollment can help you get the most from clinical trials.

Evidence-based medicine is fast becoming one of the key concepts in clinical and reimbursement practices in the developed world

Could physician behavior have a positive impact on Drug Development?

The world is suffering. But just over the horizon is a new access equation that could speed innovative vaccines to where they're needed most.

Sales reps aren't the only ones heading for the unemployment line - Pfizer is eliminating researcher positions as fewer drugs are making it to development. But could streamlining the crux of drug R&D cause more harm than good?

As global regulators set up rules for liability insurance for clinical trials, the playing field has grown complex for sponsors. This article reviews the key issues and offers a look at how European, Asian, and Latin American countries handle clinical trial insurance.

When it comes to working with clinical research sites, pharmaceutical companies should take a page out of the automakers' playbook
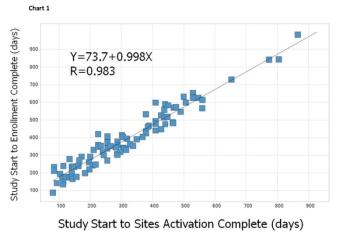
A new study of hundreds of thousands of clinical trial records reveals that the overall length of a trial is directly related to how long it takes to activate the study sites - and that most companies are wasting precious weeks and even months in activation. Here's how to improve your performance.
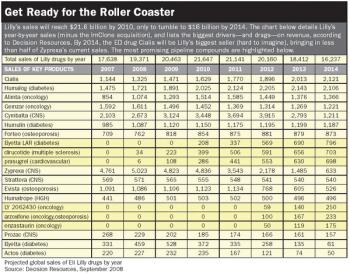
Some say it's just another pharma facing its patent cliff. But others say this company's got it worse. With new CEO John Lechleiter in charge, can Lilly find its way back from the edge?
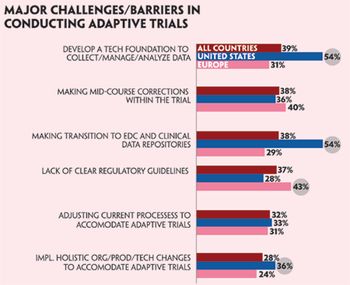
Clinical trials need to change. A recent survey shows that industry should address the research supply chain for the greatest gains in effciency.
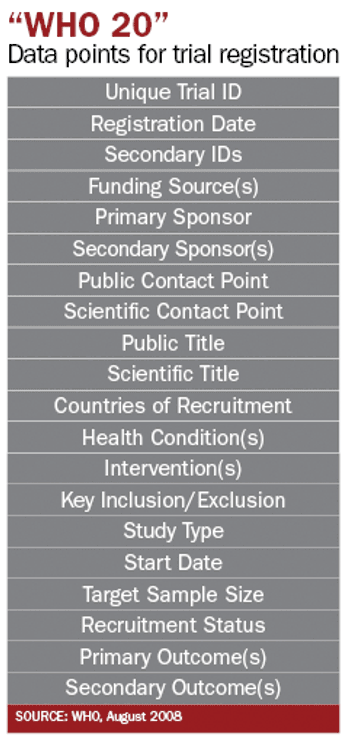
FDAAA means that you need to register clinical trials-and not only for publication planning

Concerns over quality? It's not stopping Big Pharma outsourcing and venture capital.

Roche has always gone its own way with biologics, deals, and new medicines. Now that the rest of industry has caught on, how can Roche stay ahead of its time?