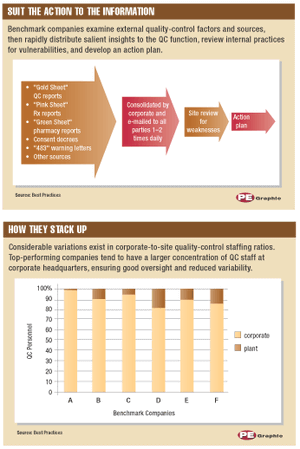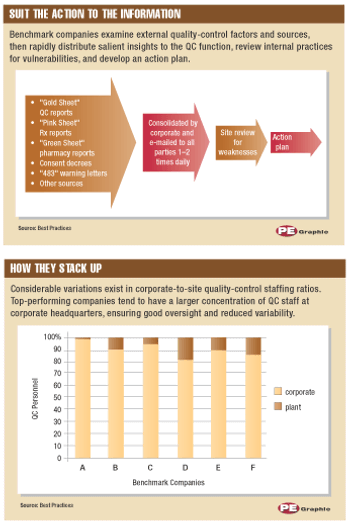
Consider the following real-world scenario: Feb. 28-Apr. 14, 2000. Third-party auditors warn Schering-Plough (SP) of problems with product quality, including lack of quality control (QC) and high staff turnover. Dec. 20, 2000. SP's stock price reaches a high of $60 per share. Jan. 19, 2001. FDA completes an in-depth inspection of SP production facilities, identifying significant, repeated, and widespread QC violations dating to 1998. Several production lines are shut down and the Clarinex (desloratidine) launch is delayed.