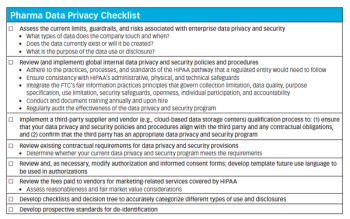
Outlining the common data transactions between life sciences companies and the HIPAA-regulated stakeholders they deal with daily-and steps pharma can take to secure and protect its own data.
Shelby Buettner is an associate at McDermott Will & Emery. She advises pharma, medical device, and healthcare companies on FDA regulatory and compliance matters.

Published: October 24th 2017 | Updated: November 15th 2020
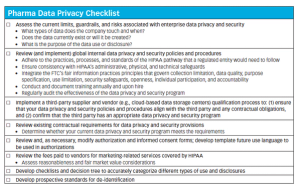
Published: November 1st 2017 | Updated: November 15th 2020