The trial size adjustment, along with protocol modifications and the addition of higher-enrollment sites, is expected to facilitate completion of the NEPHRO CRRT study of Niyad in patients undergoing renal replacement therapy by the end of 2025.

The trial size adjustment, along with protocol modifications and the addition of higher-enrollment sites, is expected to facilitate completion of the NEPHRO CRRT study of Niyad in patients undergoing renal replacement therapy by the end of 2025.
Qfitlia is the first FDA-approved antithrombin-lowering therapy indicated for routine prophylaxis to prevent or reduce bleeding episodes in patients over 12 years of age with hemophilia A or B.

Approval of Imfinzi marks the first and only perioperative immunotherapy indicated for muscle-invasive bladder cancer.
Results from the COCOON study showed that Rybrevant plus Lacluze reduced grade 2 or higher dermatologic events by 50% compared to standard care in patients with epidermal growth factor receptor-mutated non-small cell lung cancer.
Under terms of the deal, Novo Nordisk will obtain global rights to develop, manufacture, and commercialize LX9851 for obesity and metabolic disorders.
As part of the Fast Track designation, Sanofi is set to launch a Phase I/II trial to evaluate the immunogenicity and safety of its novel mRNA vaccine in preventing chlamydia.

Approval of Vykat XR marks the first treatment indicated for hyperphagia in patients with Prader-Willi syndrome.
Results from multiple clinical trials showed that Tagrisso demonstrated survival benefits in EGFR-mutated non-small cell lung cancer, both as monotherapy and in combination therapies.

Blujepa marks the first new oral antibiotic to gain FDA approval for uncomplicated urinary tract infections in nearly 30 years.
Under terms of the deal, Merck will gain global rights to develop, manufacture, and commercialize HRS-5346 for cardiovascular disease, excluding Greater China.
Under terms of the license agreement, Novo Nordisk will acquire the rights to develop and commercialize UBT251 outside of China for obesity and type 2 diabetes for an upfront payment of $200 million.

The facility, which is set to open amid Biogen’s 50th anniversary, will integrate research and development, technical operations, and commercial teams into one location.
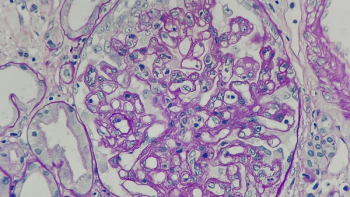
Fanhalta marks the first and only treatment for C3 glomerulopathy to gain FDA approval.

Tremfya is the first and only IL-23 inhibitor with both subcutaneous and intravenous induction options for adults with moderately to severely active Crohn disease in the United States.

The investment, which is a 25% increase from previous years, will support three new advanced manufacturing facilities, expansions at existing sites, and R&D efforts.

Results from the STEER and STRENGTH studies showed that OAV101 IT led to a 2.39-point improvement in motor function in patients with spinal muscular atrophy.
DR-0201, a CD20-directed bispecific antibody, has demonstrated robust B-cell depletion in early clinical studies, showing promise in treating refractory B-cell-mediated autoimmune diseases.
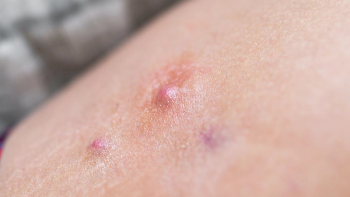
In the Phase III STOP-HS1 and STOP-HS2 trials, results show that patients treated with povorcitinib for hidradenitis suppurativa experienced a ≥50% reduction in the total abscess and inflammatory nodule count.

Latigo Biotherapeutics, Vivace Therapeutics, and Vori Health each announced the recent successful closing of their respective financing rounds.
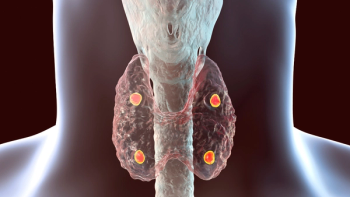
In the Phase III CALYPSO trial, eneboparatide demonstrated statistical significance in achieving albumin-adjusted serum calcium normalization while eliminating the need for active vitamin D and oral calcium therapy in chronic hypoparathyroidism.

The deals are expected to shorten manufacturing timelines for cell therapies and reduce administration times for oncology therapies, respectively.

Jonathon Whitton, AuD, PhD, VP, auditory global program head, Regeneron, discusses how the focus of technology development is shifting toward ensuring safety and continuous hearing for children.
Data from the Phase III MINT trial found that Uplizna demonstrated a greater reduction in Myasthenia Gravis Activities of Daily Living score compared to placebo at week 26.

Deal to acquire Covis is expected to expand Azurity’s portfolio across multiple complex dosage forms and key therapeutics areas.
New real-world and implementation study data highlight the efficacy of ViiV’s long-acting injectables for HIV prevention and treatment, with Apretude showing zero HIV acquisitions and Cabenuva maintaining high viral suppression rates.

Jonathon Whitton, AuD, PhD, VP, auditory global program head, Regeneron, discusses regulatory challenges for DB-OTO, Regeneron’s AAV-based gene therapy for hearing loss.
Topline results from the Phase III VERITAC-2 trial found that vepdegestrant provided a statistically significant and clinically meaningful improvement in progression-free survival in patients with ER+/HER2- advanced or metastatic breast cancer.

Partnership includes the development of combination products, including a fixed-dose combination of petrelintide and Roche’s dual GLP-1/GIP receptor agonist, CT-388, for weight management.
Results from multiple Phase III trials of Icotrokinra in moderate-to-severe plaque psoriasis and a Phase IIb trial in ulcerative colitis successfully met all primary endpoints.

Partnership is expected to support Phase III development of VK2735 in patients with obesity and metabolic disorders amid increasing demand for GLP-1 therapy.