
Aliya Omer, VP, US franchise head, breast cancer, AstraZeneca, discusses Lynparza’s role in reducing the risk of invasive disease recurrence or death in HR-positive breast cancer patients.

Aliya Omer, VP, US franchise head, breast cancer, AstraZeneca, discusses Lynparza’s role in reducing the risk of invasive disease recurrence or death in HR-positive breast cancer patients.
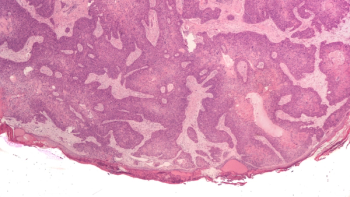
Sun Pharma’s acquisition of Checkpoint Therapeutics includes Unloxcyt, an FDA-approved anti-PD-L1 therapy for metastatic and locally advanced cutaneous squamous cell carcinoma.
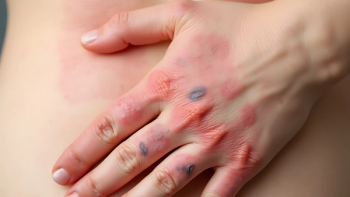
Omlyclo, a biosimilar to Xolair, is indicated for the treatment of moderate to severe persistent asthma, chronic rhinosinusitis with nasal polyps, immunoglobulin E-mediated food allergies, and chronic spontaneous urticaria.

Jonathon Whitton, AuD, PhD, VP, auditory global program head, Regeneron, discusses how key regulatory designations, such as the FDA's Regenerative Medicine Advanced Therapy designation, facilitate accelerated development and commercialization of DB-OTO through increased collaboration with regulators.
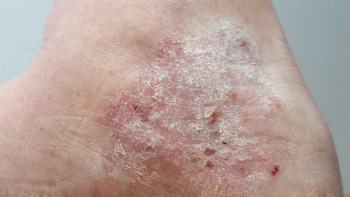
Five-year data from Phase III trials demonstrated that 67.7% of patients moderate-to-severe plaque psoriasis treated with UCB’s Bimzelx achieved complete skin clearance.

Clinical trial results found that a majority of patients with atopic dermatitis who were treated with Ebgylss achieved complete or near complete skin clearance at three years on a single monthly maintenance dose.

Jonathon Whitton, AuD, PhD, VP, auditory global program head, Regeneron, discusses the promising results of DB-OTO in the CHORD trial for children with otoferlin-related hearing loss.
The approval of neffy marks the first major advancement in epinephrine delivery for patients over four years of age in more than 35 years.
Joint venture is expected to leverage Astellas' expertise in cell therapy R&D and Yaskawa’s robotics technology to enhance accuracy, reproducibility, and efficiency in cell manufacturing.

The acquisition is expected to enhance Jazz's oncology portfolio by adding dordaviprone, a novel small molecule therapy in development for H3 K27M-mutant diffuse glioma.
Submission is supported by positive results from the Phase III REGENCY trial, which demonstrated that nearly half of patients with lupus nephritis who received Gazyva/Gazyvaro plus standard therapy achieved a complete renal response.
New indication for Tevimbra in combination with platinum-containing chemotherapy as a first-line treatment addresses an unmet need for adults with unresectable or metastatic esophageal squamous cell carcinoma whose tumors express PD-L1.
Stoboclo, a biosimilar to Prolia, is indicated for postmenopausal women and men at high risk of fracture, while Osenvelt, a biosimilar to Xgeva, is indicated for preventing skeletal-related events in patients with multiple myeloma and bone metastases from solid tumors.

Aliya Omer, VP, US franchise head, breast cancer, AstraZeneca, discusses how results of the trial compare with other treatments for gBRCAm HER2-negative high-risk early breast cancer.
In the SWIFT and ANCHOR trials, depemokimab demonstrated statistically significant reductions in nasal polyp size, obstruction, and asthma exacerbations compared to placebo.

Under terms of the deal, Gubra will receive an initial $350 million payment and could earn up to $1.875 billion more in development and sales milestones for the potential weight loss drug.

Ron Lanton, Partner, Lanton Law, discusses the legal and industry challenges posed by recent National Institute of Health budget cuts, diversity bans in clinical trials, and pharmaceutical supply chain disruptions due to tariffs.
FP008 is designed for patients with solid tumors that have not responded to existing immunotherapies targeting PD-1.

Results from a Phase III study in pediatric patients demonstrated that Odactra reduced the total combined rhinitis score by 22% compared to placebo.

Results from a Phase III registrational study suggest that ecopipam has potential as a first-in-class dopamine-1 receptor antagonist for patients with Tourette syndrome.

The Phase III SERENA-6 trial is the first global trial to use a circulating tumor DNA-guided approach to detect endocrine resistance before disease progression in HR-positive, HER2-negative advanced breast cancer.

Daniel Vitt, CEO, Immunic Therapeutics, discusses how the company is positioning itself to lead in the area of gastrointestinal diseases.

The application leverages existing clinical data for Uzedy along with prior FDA findings on the safety of Udezy in patients with bipolar I disorder.

The funding, led by existing and new investors, is expected to support Eikon’s efforts to integrate advanced computing, automation, and data science that drives innovation in drug development.

Daniel Vitt, CEO, Immunic Therapeutics, discusses how the safety and tolerabilities results from Phase I trials for IMU-856 influences further studies for gastrointestinal diseases moving forward.

Surgifort is the first FDA-approved human milk-based fortifier specifically designed for term infants recovering from gastroschisis surgery.
Keytruda demonstrated a statistically significant and clinically meaningful improvement in event-free survival and major pathological response in patients with resectable locally advanced head and neck squamous cell carcinoma.

Daniel Vitt, CEO, Immunic Therapeutics, discusses how IMU-856 could support the challenges of gluten-free diets and cross-contamination risks for celiac disease.

The BrainSense adaptive deep brain stimulation system personalizes therapy by dynamically adjusting stimulation based on real-time brain activity to improve Parkinson disease symptom control without manual adjustments.

Launching this month, Vaxitek HVT+IBD+H5 integrates COBRA technology to address the ongoing viral evolution challenges of avian influenza, as well as for the prevention of Marek disease and infectious bursal disease.