
Will Pih, co-founder, Two Labs, explains how ongoing regulatory uncertainty is slowing clinical trial activity and hiring across the industry, prompting greater reliance on specialty pharmacy services and outsourced support.

Will Pih, co-founder, Two Labs, explains how ongoing regulatory uncertainty is slowing clinical trial activity and hiring across the industry, prompting greater reliance on specialty pharmacy services and outsourced support.

Results from the Phase III MYR301 trial show that 36% of patients who achieved undetectable hepatitis delta virus levels when treated with bulevirtide maintained suppression for nearly two years.

Blake Powers, CEO, medigi, explains how digital tools, AI, and automation are streamlining access to biosimilars and cell and gene therapies, signaling a shift toward more tech-enabled, patient-centric care.

Fran Gregory, VP, emerging therapies, Cardinal Health, emphasizes the need for long-term outcomes tracking and robust health economic modeling to demonstrate the sustained value of advanced therapies and support future payment strategies.
Under terms of the deal, Alchemab will lead early Phase I trials for ATLX-1282 in amyotrophic lateral sclerosis, while Lilly will assume responsibility for further development and commercialization of the novel platform.
The Refusal to File letter comes despite the FDA’s prior encouragement to submit a supplemental biologics license application for Anktiva in patients with BCG-unresponsive non-muscle invasive bladder cancer with papillary disease.

Chip Parkinson, CEO, GiftHealth, explains how AI-powered data integration is bringing new hope to patients by revealing hidden access barriers, streamlining treatment pathways, and challenging outdated utilization management practices.

Tiara Green, president, Accessia Health, explains how empowering trusted local leaders and involving communities early are key to closing persistent gaps in preventive care and healthcare access.
Selarsdi, a biosimilar to Stelara, is approved for adult and pediatric psoriatic arthritis, plaque psoriasis, Crohn disease, and ulcerative colitis.
The 470,000-square-foot facility will support the launch and commercial production of next-generation biologics and therapies, including antibody-drug conjugates.

The Zensights Federated Healthcare Advisory Panel explores how shifting global dynamics are reshaping access and strategic planning across the healthcare system.

Will Pih, co-founder, Two Labs, explains how specialty pharmacies and manufacturers are adapting to financial pressures in rare disease care through patient-centric services, hub innovation, and hybrid technology models.

Fran Gregory, VP, emerging therapies, Cardinal Health, discusses the evolving cell and gene therapy landscape, highlighting pipeline growth, cost challenges, and emerging therapeutic areas beyond oncology and hematology.

A monthly roundup of business and people news in the pharmaceutical industry.

Doug Long, VP, industry relations, IQVIA, examines shifting pharmaceutical trends, including specialty growth, biosimilar gaps, reimbursement pressure from GLP-1s, and persistent access challenges.

Chip Parkinson, CEO, GiftHealth, explains how AI, real-time data, and closer payer collaboration are driving the next phase of growth and efficiency in specialty pharmacy.

Tiara Green, president, Accessia Health, highlights key themes from the upcoming discussion on how health equity, literacy, and social determinants are reshaping patient care—especially for those with chronic and rare conditions.

Blake Powers, CEO, Medigi, discusses how face-to-face collaboration and streamlined commercialization strategies are helping life sciences companies deliver therapies more efficiently to patients.

Investment includes $1.5 billion in capital expenditures to expand manufacturing and $500 million dedicated to R&D focused on high-impact innovations.
Approval of penpulimab-kcqx marks the company’s US regulatory debut and introduces a new immunotherapy option for advanced nasopharyngeal carcinoma.

A new study shows that varenicline combined with brief remote counseling significantly improves vaping abstinence in in patients between 16 and 25 years of age.
The submission is backed by clinical data from over 2,100 patients, including two pivotal Phase III trials in patients treated with trenibotulinumtoxinE for moderate to severe glabellar lines.

Chris Springate, CEO, ARC Medical, discusses how postoperative adhesions continue to cause chronic pain, infertility, and life-threatening complications—despite advances in surgical techniques and often going undiagnosed for years.
In the Phase III HARMONi-6 trial, ivonescimab plus chemotherapy significantly outperformed tislelizumab plus chemotherapy as a first-line treatment for advanced squamous non-small cell lung cancer.
Results from the dual Phase III TRIUMpH trials demonstrated the statistical superiority of tegoprazan over lansoprazole in patients with erosive esophagitis and non-erosive reflux disease.

In the Phase III ARISE trial, Cobenfy administered as an adjunctive treatment to atypical antipsychotics for patients with inadequately controlled schizophrenia did not achieve statistically significant improvements.
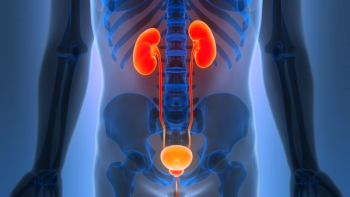
Results from the Phase IIb SunRISe-1 study demonstrated a one-year duration of response with the TAR-200 intravesical gemcitabine-releasing system in patients with Bacillus Calmette-Guérin-unresponsive high-risk non-muscle-invasive bladder cancer.

With combined investments exceeding $53 billion, both companies are deepening their US presence through expanded biologics production, gene therapy capabilities, and next generation R&D centers.

Nigel McCracken, chief operating officer, Virax Biolabs, discusses the expansion of its ViraxImmune platform into areas such as transplant monitoring, vaccine efficacy, latent virus reactivation, and CAR T cell therapy.

The simplified requirements for Camzyos are expected to ease prior contraindications, thereby simplifying treatment and expanding eligibility in patients with symptomatic New York Heart Association Class II-III obstructive hypertrophic cardiomyopathy.