
App aims to leverage digital technology to significantly reduce pharmacy costs.

App aims to leverage digital technology to significantly reduce pharmacy costs.

In an interview with Pharm Exec associate editor Don Tracy, Lynn Kirkpatrick, PhD, CEO, Ensysce Biosciences, discusses the FDA's Breakthrough Therapy Designation for PF614-MPAR, which focuses on protecting patients from drug overdoses.

Eli Lilly advertisement emphasizes that advancements in in medicine can lead to improved population health.

In an interview with Pharm Exec Associate Editor Don Tracy, Ron Lanton, Partner, Lanton Law PLLC, discusses recent moves by CVS and Express Scripts to follow Mark Cuban's Cost Plus Drugs model.
Atypical antidepressant linked to severe adverse effects and death as a result of misuse.

In an interview with PharmExec Associate Editor Don Tracy, Jingsong Wang discusses Nona Biosciences’ recent exclusive license agreement with Pfizer Inc. for the development and commercialization of HBM9033.

Report comes after the FDA seized thousands of counterfeit Ozempic units.
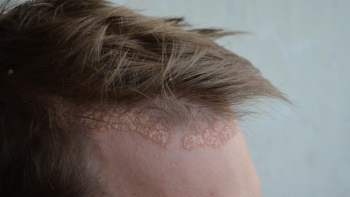
Phase IIIb trial of Tremfya demonstrated rapid improvements in multiple areas of scalp psoriasis at 16 weeks.
New FDA guidelines require manufacturers to add boxed warnings to CAR T-cell therapy products.

Offerings include treatment plans for a considerable number of illnesses and conditions, company says.
NK010 is reportedly the first Chinese trial of its kind to be approved by the FDA.
Opdivo plus Yervoy shows promise in treating patients with microsatellite instability-high or mismatch repair deficient metastatic colorectal cancer.

Study reportedly demonstrates the encouraging safety profile of satri-cel, the first autologous Claudin18.2 CAR T cell therapy.
Bloomlife’s MFM-Pro is a prescription-based wearable device that can track the maternal and fetal heart rate.

Researchers believe low numbers in the use of direct-to-consumer healthcare services could rapidly trend up.

Company displayed findings at the 2024 Winter Clinical Dermatology Conference in Hawaii demonstrating significant improvements in atopic dermatitis and seborrheic dermatitis.

Roots Analysis report suggests increase can be attributed to the growing demand for advanced therapies and biologics.

In an interview with Pharmaceutical Executive associate editor Don Tracy, Ashley Gaines, VP, head of breast cancer franchise, discusses newly approved Truqap.

JAMA study investigates whether physical or behavioral healthcare needs are associated with the risk of underinsurance across household income levels.

Company cites potential safety risks for patients who ignore the intended indication of Mounjaro and Zepbound.

JAMA study evaluates the ability of artificial intelligence to transform electronic health.

Oxford Academic report explores the positive improvements these technological capabilities could bring to the industry.

American Heart Association survey suggests holiday stress has a major impact on health habits.
JAMA study aims to discover whether ignoring medical advice relates to financial status among patients with heart failure.
JAMA Network study evaluates current attitudes toward individuals with acne with a call for pharma companies to focus efforts on helping to overcome these stigmas.
PREPVACC study expected to stop further HIV vaccination attempts after previously enrolling 1,500 participants in East and Southern Africa.

Camille Lee explains the ins and outs of the patient support system for psoriasis medication.

UCB executive speaks on recent FDA approval of Bimzelx.
National Library of Medicine study emphasizes inadequacies in ensuring the safety and efficacy of over-the-counter dietary supplements.

An overview of the nine biggest developments to expect across the healthcare industry in the year ahead.